Ruuhfwlrqv – IKA C 5000 control Package 2/12 User Manual
Page 14
&DORULPHWULF PHDVXUHPHQWV
IKA
-WERKE C 5000 control/duo-control Ver. 10 04.07
3DJH
Often, however, the products of combustion assumed by the standards are not the
only ones that are formed. In such cases, analyses must be performed on the fuel
sample and the combustion products that yield data for a correction calculation. The
standard gross calorific value is then determined from the measured gross calorific
value and the analysis data.
The Ho gross calorific value is formed from the quotient of the quantity of heat liber-
ated during complete combustion of a solid or liquid combustible substance and the
weight of the fuel sample. In this calculation, the water formed before the combus-
tion of compounds of the combustible substance must be present in a liquid state
after the combustion.
Reference temperature 22°C
The net calorific value Hu is equal to the gross calorific value reduced by the energy
of condensation of the water that was contained in the combustible substance that is
formed by combustion. The net calorific value is the technically more important
quantity, since only the net calorific value can be evaluated in terms of energy in all
important, technical applications.
On the calculation formulas for gross and net calorific value, see Section 17 “Basic
of calculations”.
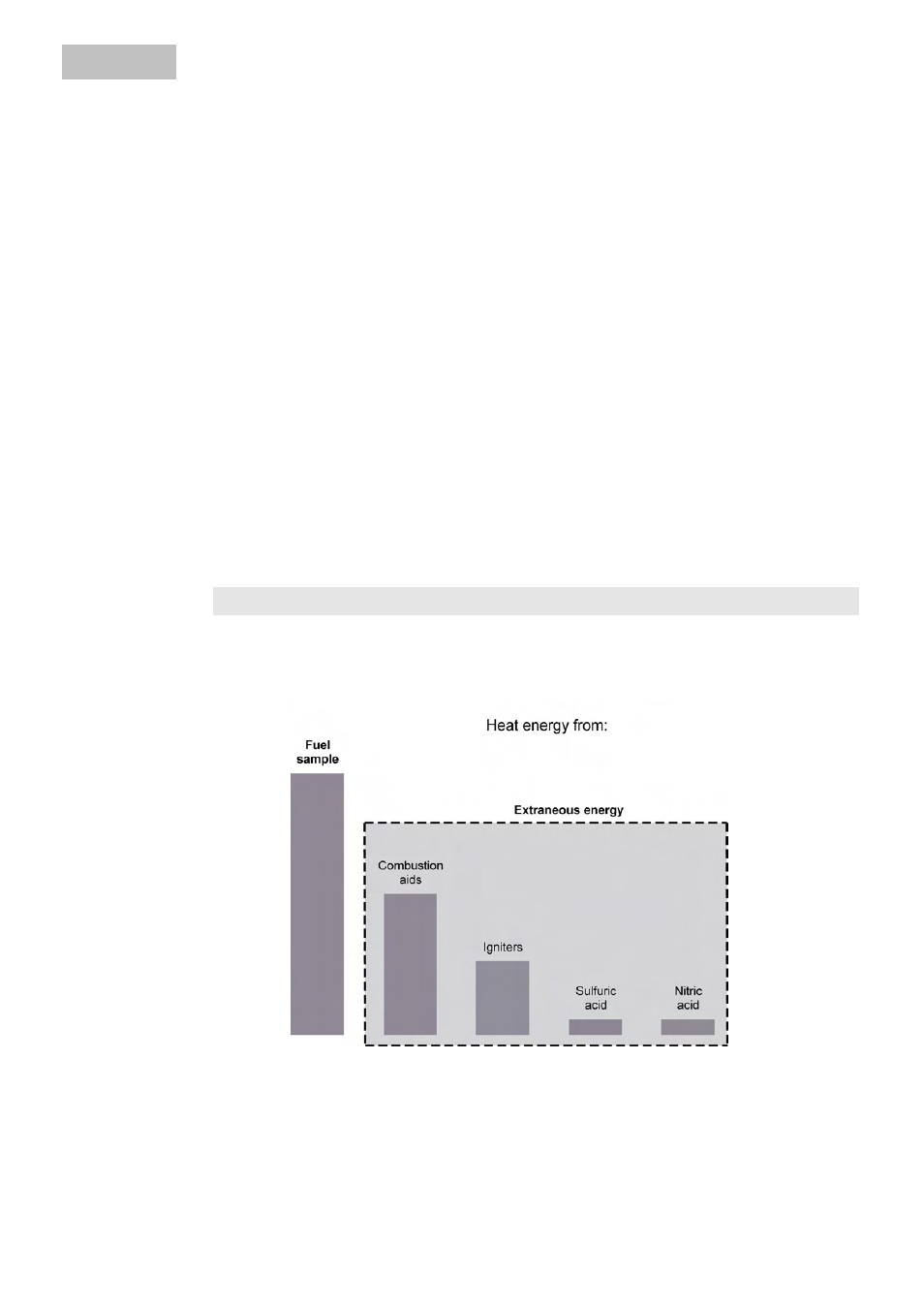
&RUUHFWLRQV
During a combustion experiment, as conditioned by the system, heat is not gener-
ated only by combustion of the sample; in addition heat also arises through extrane-
ous energy:
The heat of combustion of the cotton thread that ignites the sample and the heat of
electrical ignition would distort the measurement. This effect is taken into account in
the calculation with a correction value.
Substances with low inflammability and substances that do not readily undergo
combustion are burned together with a combustion aid. The combustion aid is first
weighed and is then placed in the crucible with the sample. From the weight of the
+R JURVV
FDORULILF YDOXH
+X QHW FDORULILF
YDOXH
,JQLWHU
&RPEXVWLRQ
DLG
¢¡¤£¦¥¨§©¨¤§¤¤¥
§!"£#!%$
¡¦&¤¥ '
£#!%¡%§¨¤"¡#!%¡#')(#0#1
The extraneous energy
can vary considerably
in relation to the heat of
combustion of the fuel
sample.
