PASCO CI-6729 (1X) CONDUCTIVITY SENSOR User Manual
Page 7
3
012–06485B
Conductivity Sensor
Theory—Principles of Operation
of the Conductivity Sensor
What is Conductivity?
Conductivity (or specifically, electrolytic conductivity) is defined as the ability
of a substance to conduct electric current. It is the reciprocal of the more
commonly encountered term, resistivity. All substances possess conductivity
to some degree, but the amount varies widely, ranging from extremely low
(insulators such as benzene and glass) to very high (silver, copper, and metals
in general). Most industrial interest is in the conductivity measurement of
liquids that generally consist of ionic compounds dissolved in water. These
solutions have conductivities approximately midway between insulators and
metallic conductors. This conductivity can be measured quite easily by
electronic means, allowing a simple test that can tell much about the quality
of the water or the makeup of the solution. A broad line of conductivity
equipment is available to measure liquids ranging from ultrapure water (low
conductivity) to concentrated chemical streams (high conductivity).
Units of Conductivity
The units of measurement used to describe conductivity and resistively are
quite fundamental and are frequently misused. Once the units are known,
various waters can be quantitatively described.
The basic unit of resistance is the familiar ohm. Conductance is the
reciprocal of resistance, and its basic unit is the siemens (S), formerly called
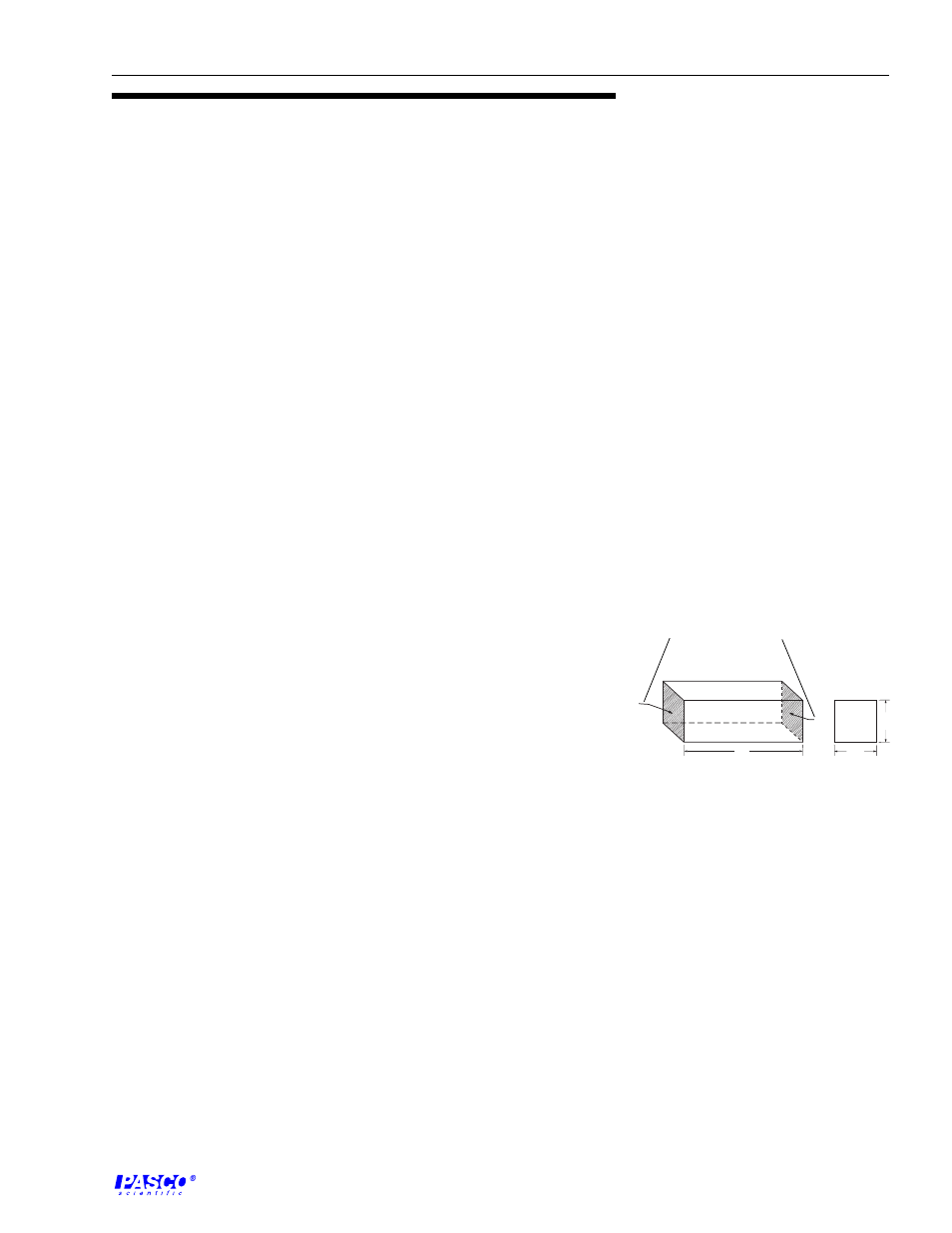
mho. In discussions of bulk material, it is convenient to talk of its specific
conduct-ance, now commonly called its conductivity. This is the conductance
as measured between the opposite faces of a 1 cm cube of the material
(Figure 1).
This measurement has units of siemens/centimeter (S/cm). The units
microsiemens/centimeter (µS/cm) and millisiemens/centimeter (mS/cm)
are most commonly used to describe the conductivity of aqueous solutions.
The corresponding terms for specific resistance (or resistivity) are
ohm - centimeter (
Ω · cm), megaohm - centimeter (M Ω · cm) and
kiloohm - centimeter (k
Ω · cm).
Users of ultra pure water prefer to use resistivity units of M
Ω · cm, because
measurement in these units tends to spread the scale out in the range of
interest. These same users frequently use k
Ω · cm when dealing with less
pure water such as tap water.
B
L
Pt
Pt
A
A
W
H
Figure 1
Conductivity Cube
conducting surface
L
A
