PASCO CI-6729 (1X) CONDUCTIVITY SENSOR User Manual
Page 24
20
Conductivity Sensor
012–06485B
the solution will increase. Students can use a magnetic stirrer, if
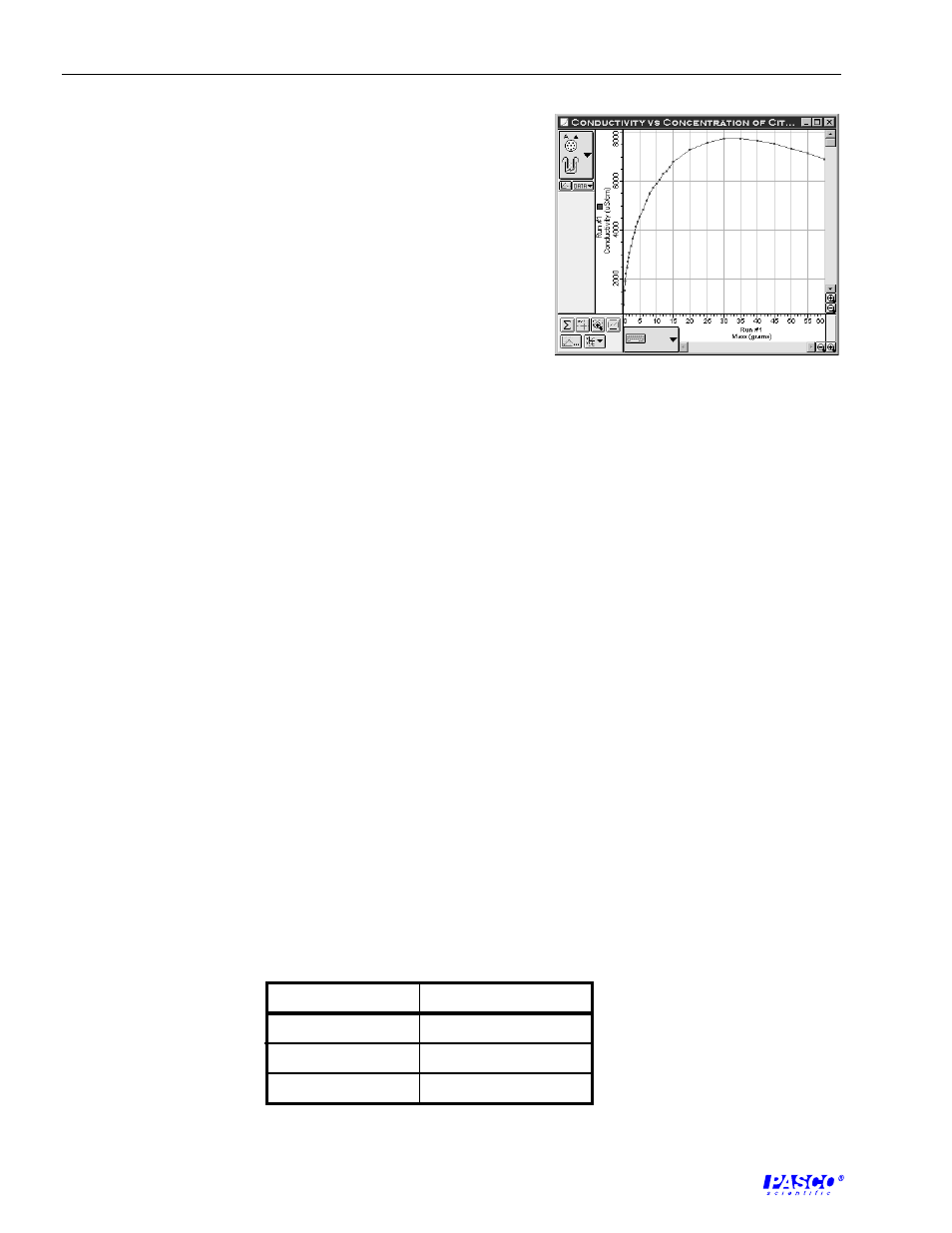
available. An example of the data is shown in Figure TG-1.
Answers to Questions
1. Students should find that at low concentrations the
conductivity increases linearly with added mass of the citric
acid. At higher concentrations the conductivity will begin
to deviate from linearity and eventually decrease when the
concentration is very high.
2. As the concentration increases, the electrolytes are more
likely to collide. Therefore, their mobility decreases.
Furthermore, the degree of dissociation of the solvent
decreases as the solution approaches saturation. As the
solution approaches saturation, the concentration dependence of conductivity deviates from
linearity. At low concentrations the mobility of the ions and the degree of dissociation of the
molecules remain constant, thus the dependence is linear.
Notes on Experiment 3
It is assumed that the student has completed experiments 1 and 2 before carrying out this
experiment.
1. If bubbles form inside the conductivity cell, the conductivity reading will be reduced, since
the they will form an insulating layer on one or both of the cell electrodes. This is more
likely to occur at higher temperatures. One can eliminate the bubbles by increasing the
setting on the magnetic stirrer to allow the solution to flow through the cell. This effect can
also be eliminated by tapping/shaking the electrode.
2. If time is limited, the solutions could be prepared before the laboratory period begins.
Data Analysis
The table below lists typical experimental results. In general, ionic salts at low to moderate
concentrations have a temperature dependence of 2% / °C at 25 °C. Acids, bases, and
concentrated salt solutions have somewhat lower values, typically 1.5% / °C. On the other
hand, ultra pure water has by far the largest slope, 5.2% / °C.
Answers to Questions
2.1
2.0
1.9
Solution
percent/ °C at 25 °C
1000 ppm NaCl
4000 ppm NaCl
1000 ppm NaOH
Figure TG-1
