PASCO CI-6729 (1X) CONDUCTIVITY SENSOR User Manual
Page 22
18
Conductivity Sensor
012–06485B
8. Rinse the 50 ml buret with a few ml of the 0.5 M sodium
hydroxide (NaOH) solution. Dispose of the rinse solution as
directed.
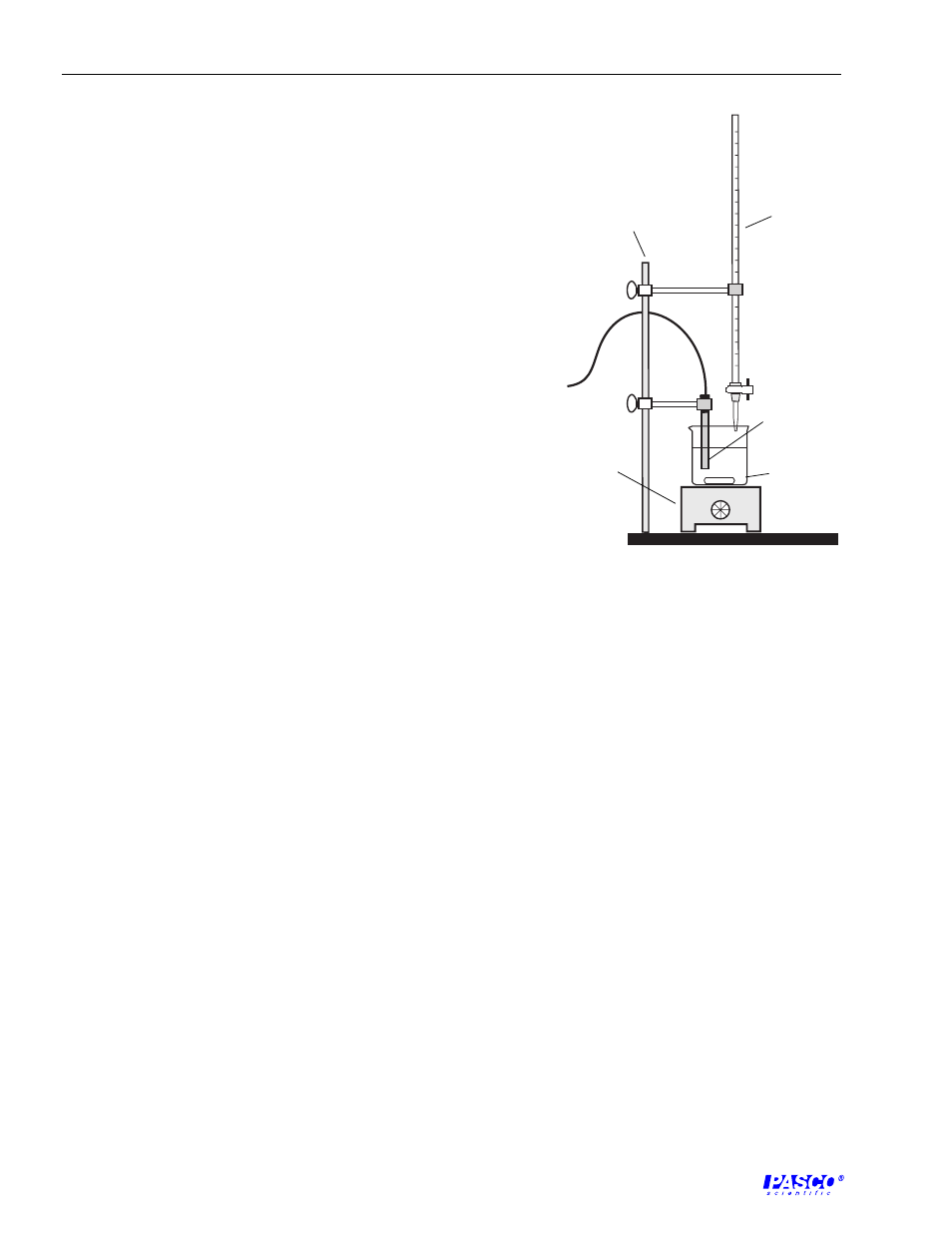
9. Use a second buret clamp to attach the buret to the support
rod. Arrange the buret above the beaker so that you can use
the buret to add precise amounts of solutions to the beaker
(Figure 4.1).
10. Fill the buret with 0.5 M NaOH solution to the 0 mark on the
buret. Be sure to start the titration with the buret filled with
0.5 M NaOH solution and the volume used at exactly 0.00
ml.
11. Turn on the magnetic stirrer.
12. Click REC. (The Keyboard Sampling window will open.
Move the window so you can see the other displays.) Do
not add any titrant for the first reading. In the Keyboard
Input box, type 0.00 for Entry #1 and click Enter.
13. Add 0.5 M NaOH to the 0.03 M HCl solution in increments
of 1 ml. In the Keyboard Input box, type the total volume of
titrant added to the HCl solution, and click Enter. (Make sure the reading on the Digits
display stabilizes first.)
14. Repeat step 13 until 5 ml of titrant is added.
15. After 5 ml of titrant has been added, add 3–10 drops of 0.5 M NaOH per increment.
Decrease your increments as you approach the equivalence point. Note that 3 drops is
approximately the smallest increment that will yield a noticeable change in the volume of
titrant added to the solution. As you approach the equivalence point, you will see patches of
pink in the solution for each drop of titrant added. Continue to type in the total volume of 0.5
M NaOH added after each increment. Observe the values of the conductivity near the
equivalence point.
16. After approximately 5 ml of 0.5 M NaOH is added past the equivalence point, increase
your increments to 1 ml larger, and continue typing the total volume of titrant added.
17. When the conductivity reaches 15,000–20,000
µs/cm, click Stop Sampling and turn off the
magnetic stirrer. Rinse the sensor with the wash bottle.
(Optional) Repeat the experiment using 150 ml 0.03 M acetic acid.
Questions
1. Based on your observations, how can the Conductivity Sensor be used to determine the
equivalence point?
2. How much 0.5 M NaOH titrant was required to reach the equivalence point?
Figure 4.1
Experiment Setup
conductivity
electrode
beaker &
spin bar
magnetic
stir plate
buret
rod, support
stand, and
buret clamps
