Bio-Rad Experion Protein Analysis Kits User Manual
Page 12
1.2.6 Prepare the Samples and the Pro260 Ladder
1.
Prepare the Pro260 ladder. Mix 4 µl Pro260 ladder (red cap) and 2 µl sample buffer
“R” (reducing sample buffer) in a 0.65 ml microcentrifuge tube. Vortex the tube briefly
and spin down for a few seconds. Label the tube “L”.
2.
Prepare the samples (S1–S3). Label three 0.65 ml microcentrifuge tubes “S1”, “S2”,
and “S3”. For each sample, combine 4 µl of the BGG samples in tubes 1–3 (from
Section 1.2.4) with 2 µl sample buffer “NR” (nonreducing).
3.
Vortex all tubes briefly and spin down in a microcentrifuge for a few sec.
4.
Place the sample tubes S1–S3 and the Pro260 ladder (L) in a 95–100°C heating block
for 3–5 min.
5.
Spin the tubes for 15 sec, add 84 µl DEPC-treated water to each tube, and vortex
briefly to mix.
1.2.7 Prime the Chip
Before priming the chip, be sure that all samples, the Pro260 ladder, and other reagents
are prepared and ready to be loaded. If a chip is not used within 5 min of priming and
loading, reagents may evaporate, leading to poor results. Use the narrow-bore pipet tips
that are supplied with the kit (or equivalent tips) when loading samples and reagents into
the chip.
1.
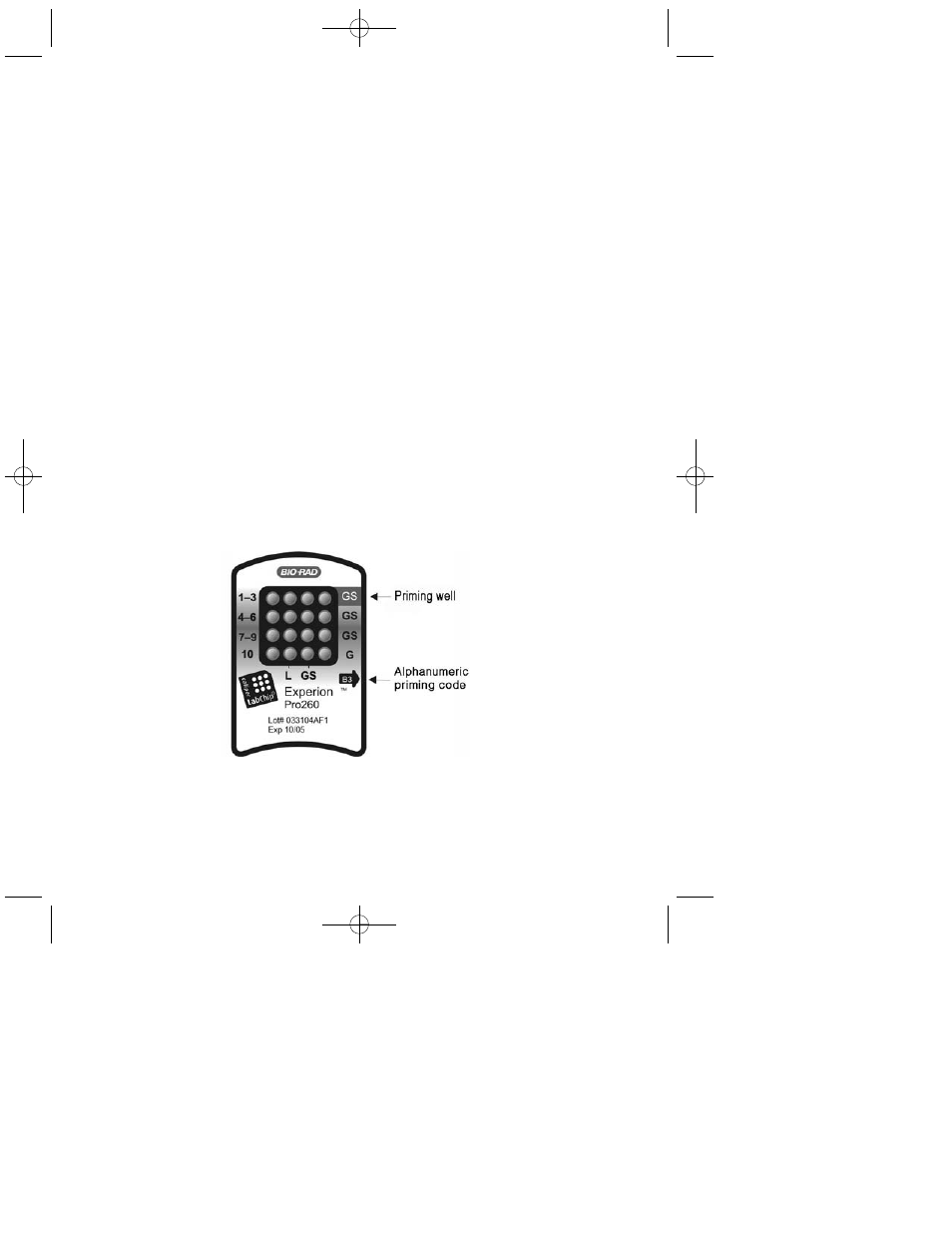
Pipet 12 µl GS into the top right well labeled GS (gel priming well) (Figure 1.1). Insert
the pipet tip vertically and to the bottom of the well when dispensing. Dispense slowly,
and do not expel air at the end of the pipetting step.
Note: Placing the pipet tip at the edge of the well or allowing the gel to slide down the wall
of the well may lead to bubble formation at the bottom of the well. Dislodge any bubbles at
the bottom of the well with a clean pipet tip, or remove the GS and load it again. For help
with chip loading, refer to the Experion Training Video: Chip Loading.
Fig. 1.1. Experion Pro260 chip. The locations of the gel priming well (GS) and alphanumeric priming
code are indicated.
8
10010510A.qxp 2/13/2008 11:43 AM Page 8
