Technical data, Technical data 6.1 measuring principle – KROHNE OPTISENS COND 1200 EN User Manual
Page 36
6
TECHNICAL DATA
36
OPTISENS COND 1200
www.krohne.com
01/2014 - 4001967301 - MA OPTISENS COND 1200 R01 en
Technical data
6.1 Measuring principle
6.1.1 Conductive measurement
The principle of conductivity measurement is defined as the capacity of a solution to conduct an
electrical current between two electrodes. For determining the electrolytic conductivity it is
necessary to record the number of dissolved ions summarily. The parameter serves as a scale
for water purity and is given in Siemens. As there are two open cells, mutual voltage is being
produced. This one on its part generates electricity depending on the resistance of the medium.
As the medium is in direct contact with the electrode, the medium reacts faster to differences in
measuring values. The integrated temperature sensor compensates the conductivity.
Using Ohm’s law: Ohm = Voltage/Current, the resistance of a liquid can be determined by
measuring the current while keeping voltage constant. Specific conductivity is defined by
1/resistance. The unit of measurement is Siemens and is normally expressed in μS/cm or
mS/cm. An important criterion for the measuring range of conductivity cells is the geometry of
the electrodes. There are two rules which are characteristic for conductivity measurement:
1. The larger the distance between the two electrodes, the larger the resistance.
2. The larger the electrode surface, the lower the resistance.
The surface area (A) and the distance (L) must be correctly matched to the desired measuring
range. This is called the "cell constant" defined as c=L/A.
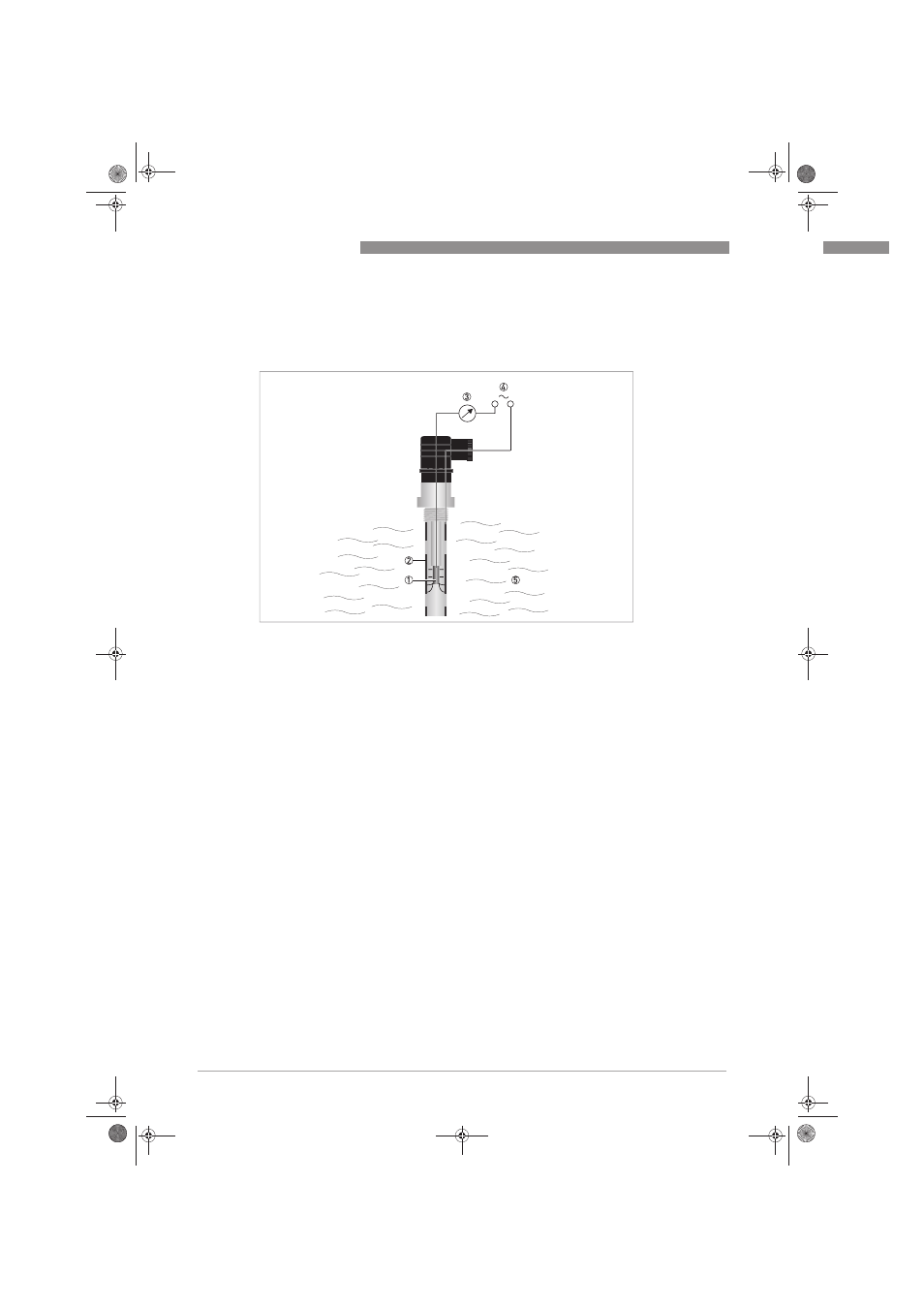
Figure 6-1: Measuring principle for conductivity measurement
1 Inner electrode
2 Outer electrode
3 Current measurement
4 Power supply
5 Measuring medium
.book Page 36 Friday, January 24, 2014 12:32 PM
