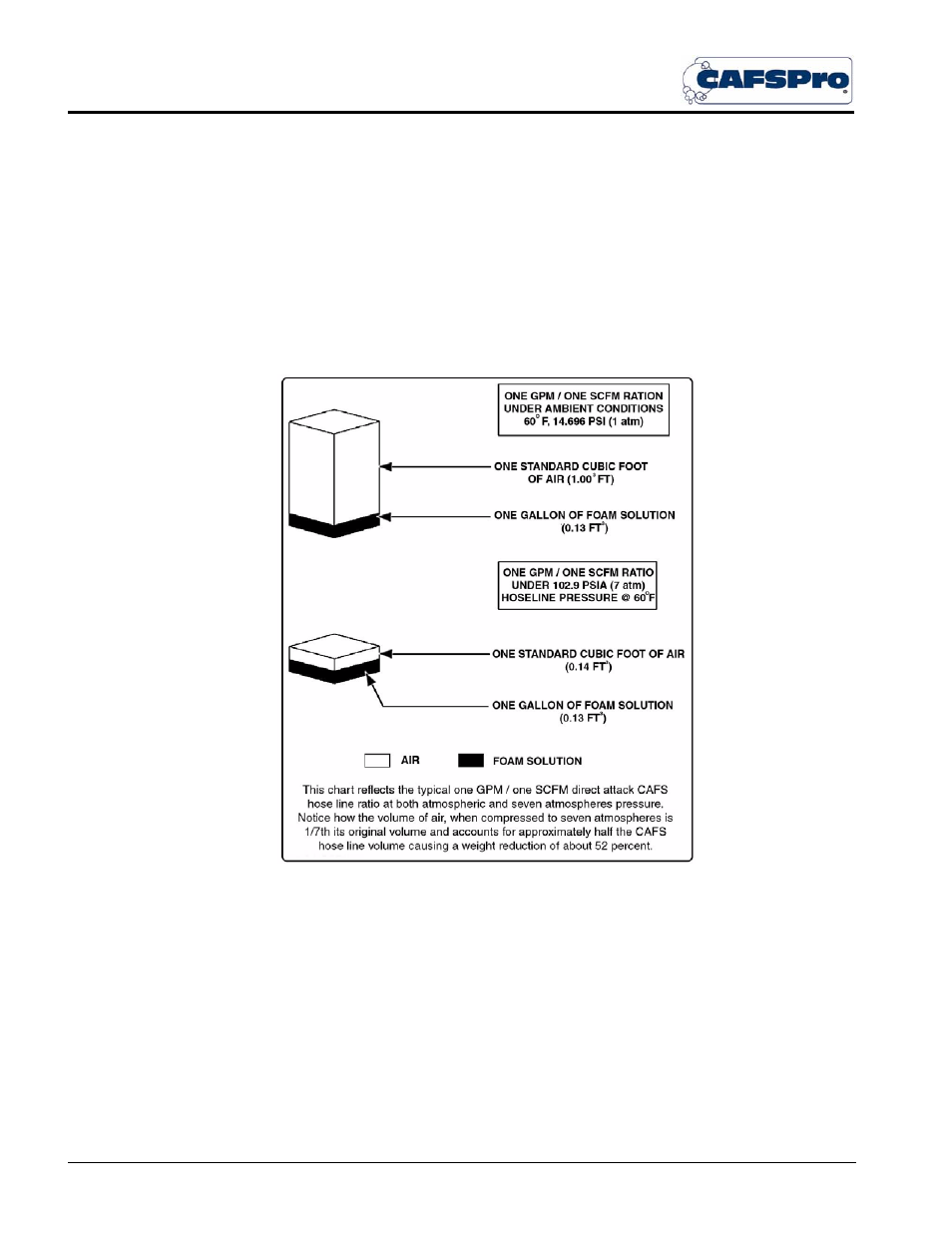
Figure 2-3: boyle’s law - schematic representation – Hale CAFSPro User Manual
Page 16
❑ Overview
16
CAFSPro User Operation Manual
p/n: 029-0020-75-0
❑
Nozzle Reaction/Compressibility of Air
Compressed air foam hose lines, by definition, contain a mixture of com-
pressed air, foam and water. Since compressed air stores energy, a
surge is felt when opening the nozzle as the air escapes.
Open the nozzle slowly to minimize this surge. Also see Section “1
Safety Precautions” on page 7 for additional nozzle reaction information.
To better understand nozzle reaction and the high energy nature of compressed air
foam, the compressibility of air as defined by Boyle’s Law can be studied.
Boyle’s Law
states: If the
temperature is
kept constant, the
volume of a gas
will vary inversely
as the absolute
pressure, while
the density will
vary directly as
the pressure.
Since the pres-
sure and volume
of a gas are
inversely related
— the higher the
pressure, the
smaller the vol-
ume, and vice
versa.
Figure 2-3: Boyle’s Law -
Schematic Representation
The formula for
Boyle’s law is: PV=C.
Where P = Absolute Pressure; V = Volume and C = Constant
Figure 2-3: “Boyle’s Law - Schematic Representation“ shows that the origi-
nal volume of compressed air is reduced as the pressure increases by a
directly proportionate factor.
As the schematic shows, with six more atmospheres [88.2 PSI (6 BAR)] of
pressure added, the air pressure is now seven atmospheres [102.9 PSI (7
BAR)], and the original volume has been reduced by a factor of one-seventh
(1/7). As more pressure is added, each atmosphere [14.7 PSI (1 BAR)] of
pressure directly reduces the volume of air by the factor shown.
