Jenway 7315 Manual Italian User Manual
Page 16
16
SeCtion 3 – theory and Practice of Spectroscopy Measurements
3.1
tHeoRY oF SPeCtRoSCoPY MeASUReMent
UV-visible spectroscopy is the measurement of the absorbance of light at a specific wavelength in a
sample. This is used to identify the presence and concentration of molecular entities within the sample.
The Beer-Lambert law is used to relate the absorption of light to the properties of the sample through
which the light is travelling through. The Beer-Lambert law states that:
A
is the absorbance
is the molar absorption coefficient (l mol
-1
cm
-1
)
c
is the concentration (mol l
-1
)
l
is the path length (cm)
This law shows that absorbance is linear to concentration but this is only true for low concentrations. For
absorbance levels above 3 the concentration starts to move away from the linear relationship.
Transmittance is the proportion of the light which passes through the sample:
Therefore: T = I
t
I
o
Absorbance is inversely related to transmittance:
A = log 1
T
3.2
SPeCtRoSCoPY MeASUReMent
There are four main components of a spectrophotometer. These are a light source to emit a high and
constant amount of energy over the full wavelength range; a method for separating the light into
discreet wavelengths; a sample holder and a light detector.
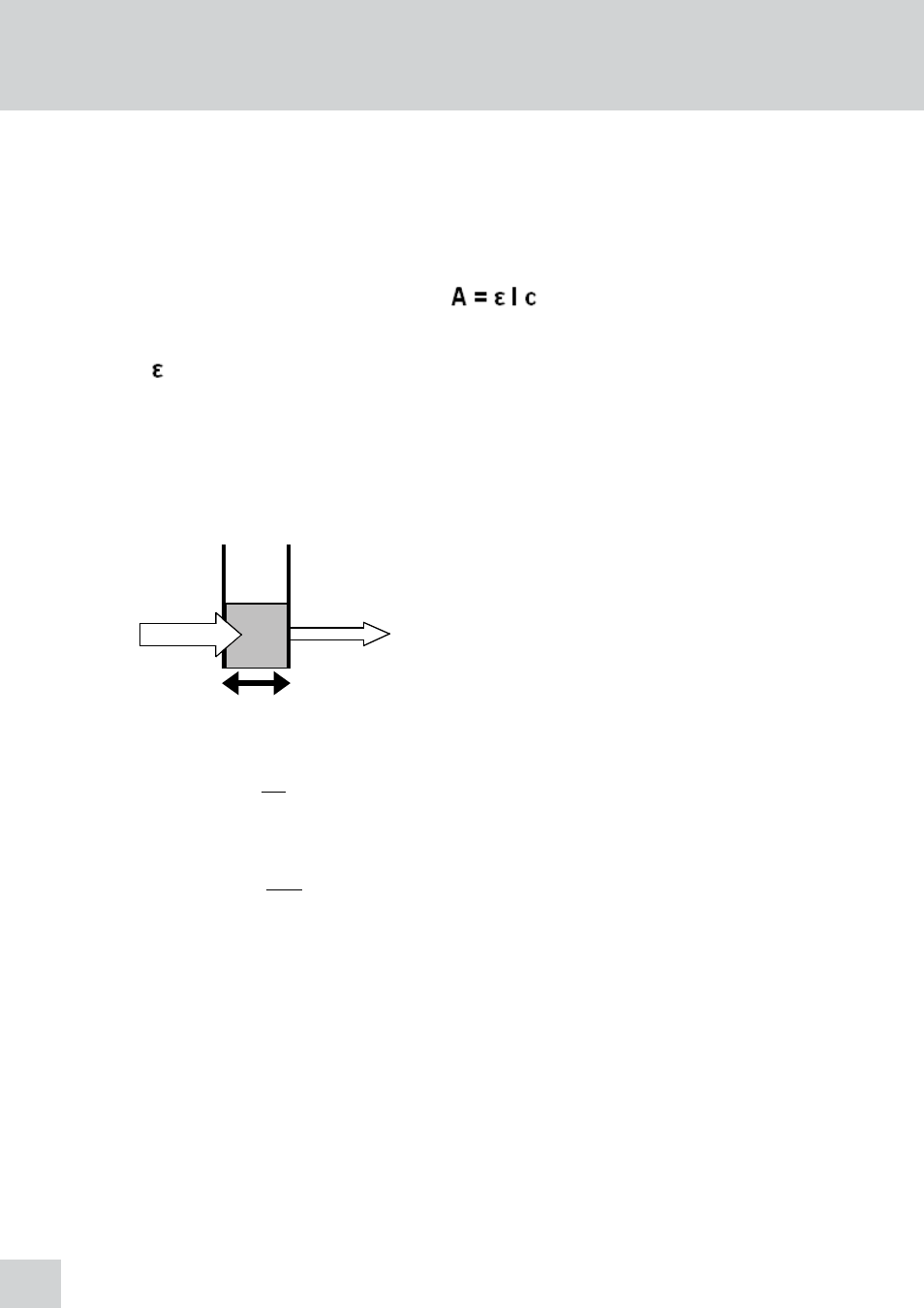
Where:
L
o
is the incident light
l
t
is the transmitted light
l is the path length
l
I
o
I
t
l
