8 orp (oxidation-reduction potential), 8 orp (oxidation-reduction potential) -9 – Yokogawa PH72 Personal pH/ORP Meter User Manual
Page 76
IM 12B03D02-01E
9-9
9. Technical Information
9.8 ORP (Oxidation-Reduction Potential)
In general, oxidation is the gain of oxygen or the loss of hydrogen, and reduction is the
loss of oxygen or the gain of hydrogen. In the electrochemistry field, oxidation is defined
as the loss of electrons and reduction is defined as the gain of electrons. These reactions
are reversible and expressed as follows:
Ox
؉ n e Red
-
where Ox is the oxidized form of substance, Red is the reduced form of substance, e
–
is
an electron, and n is the number of electrons transferred. If an inert electrode (not react
with substances in a solution or not corroded by a solution, e.g., platinum or gold) is
immersed in a solution where oxidized and reduced forms of substances are present, the
electrode will acquire the potential that corresponds the ratio of activities of both forms
of substances and reaches its equilibrium. This potential is called the oxidation-reduction
potential (ORP). The ORP, E in millivolts, between the indicator electrode and the
reference electrode is expressed from the Nernst equation as follows.
E : oxidation-reduction potential when potential of
standard hydrogen electrode* is 0
E : standard electrode potential when [Ox]
؍[Red]
R : gas constant
F :
Faraday constant
n
: number of electrons
T : absolute temperature
[Ox] : activity of oxidized form of substance
[Red] : activity of reduced form of substance
E
؍ E ؉
ln
R T
n F
[Ox]
[Red]
*
؇
؇
Standard Hydrogen Electrode (SHE)
Where:
(9.6)
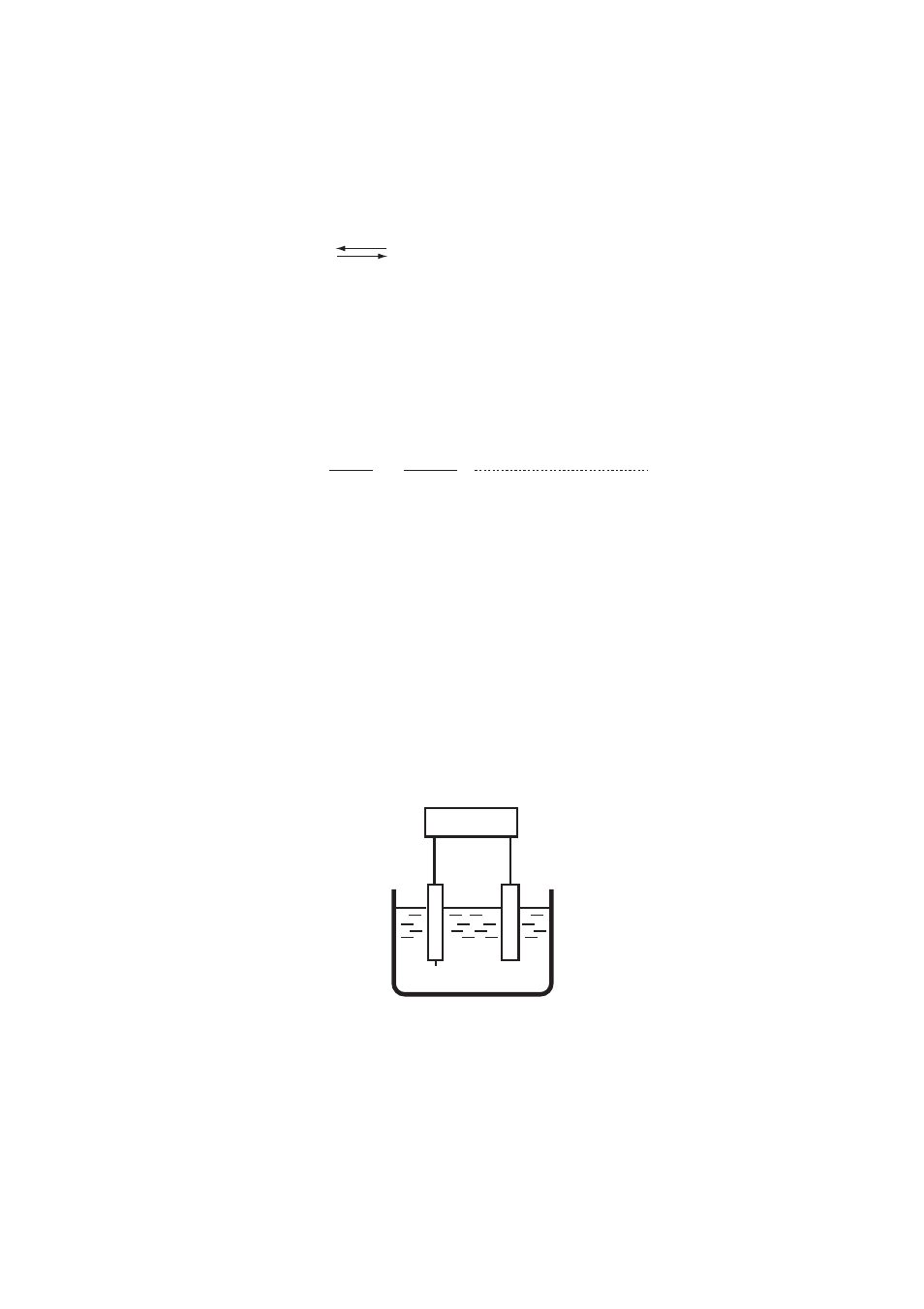
F0911.EPS
Potentiometer
Test solution
Reference electrode
Indicator electrode (Pt)
E
Figure 9.11
Measurement System of ORP Meter
