4 the asymmetry potential, 4 the asymmetry potential -5 – Yokogawa PH72 Personal pH/ORP Meter User Manual
Page 72
IM 12B03D02-01E
9-5
9. Technical Information
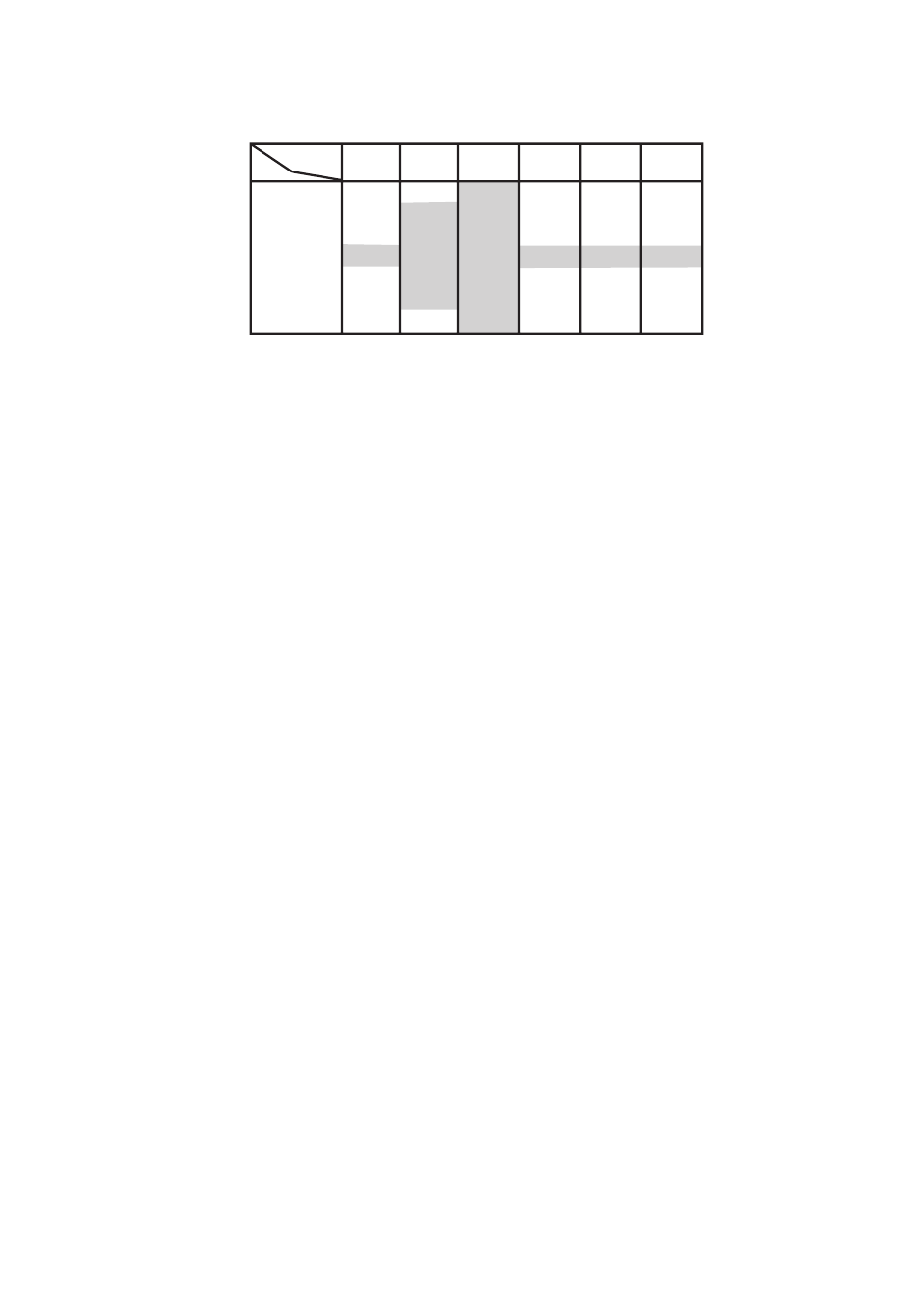
Table 9.2
Deviations from True Values in Measurement without
Temperature Compensation
1
3
5
7
9
11
13
0.50
0.34
0.17
0.00
-0.17
-0.34
-0.50
0
Temp.(
؇
C)
pH
0.10
0.07
0.03
0.00
-0.03
-0.07
-0.10
20
0.00
0.00
0.00
0.00
0.00
0.00
0.00
25
-0.30
-0.20
-0.10
0.00
0.10
0.20
0.30
40
-0.70
-0.47
-0.23
0.00
0.23
0.47
0.70
60
-1.11
-0.74
-0.37
0.00
0.37
0.74
1.11
80
T0902.EPS
In addition, the pH value of a solution changes with temperature. The pH value of a
solution at the actual temperature may be converted into the one at a reference
temperature. This is generally called “conversion to reference temperature,” which is
different from the temperature compensation.
9.4 The Asymmetry Potential
Theoretically when identical buffer solutions (pH
i
= pH
S
) are present on both sides of the
membrane of a glass electrode, the emf should be 0 mV. In reality, some potentials (C
S
–
C
i
) develop depending on the thickness of the glass membrane, heat treatment process,
service history, or other factors. This is called the real asymmetry potential. In addition
to this potential, the difference in single electrode potential between the inner electrodes
of the glass electrode and of the reference electrode and a liquid junction potential* are
collectively referred to as the apparent asymmetry potential or just the asymmetry
potential. This asymmetry potential is E
AS
in equation 9.4.
* Liquid junction potential occurs due to dirt or clogging of the liquid junction or
other factors.
