PASCO PS-2108 Dissolved Oxygen Sensor User Manual
Page 6
PASPort Dissolved Oxygen Sensor
Model No. PS-2108
2
®
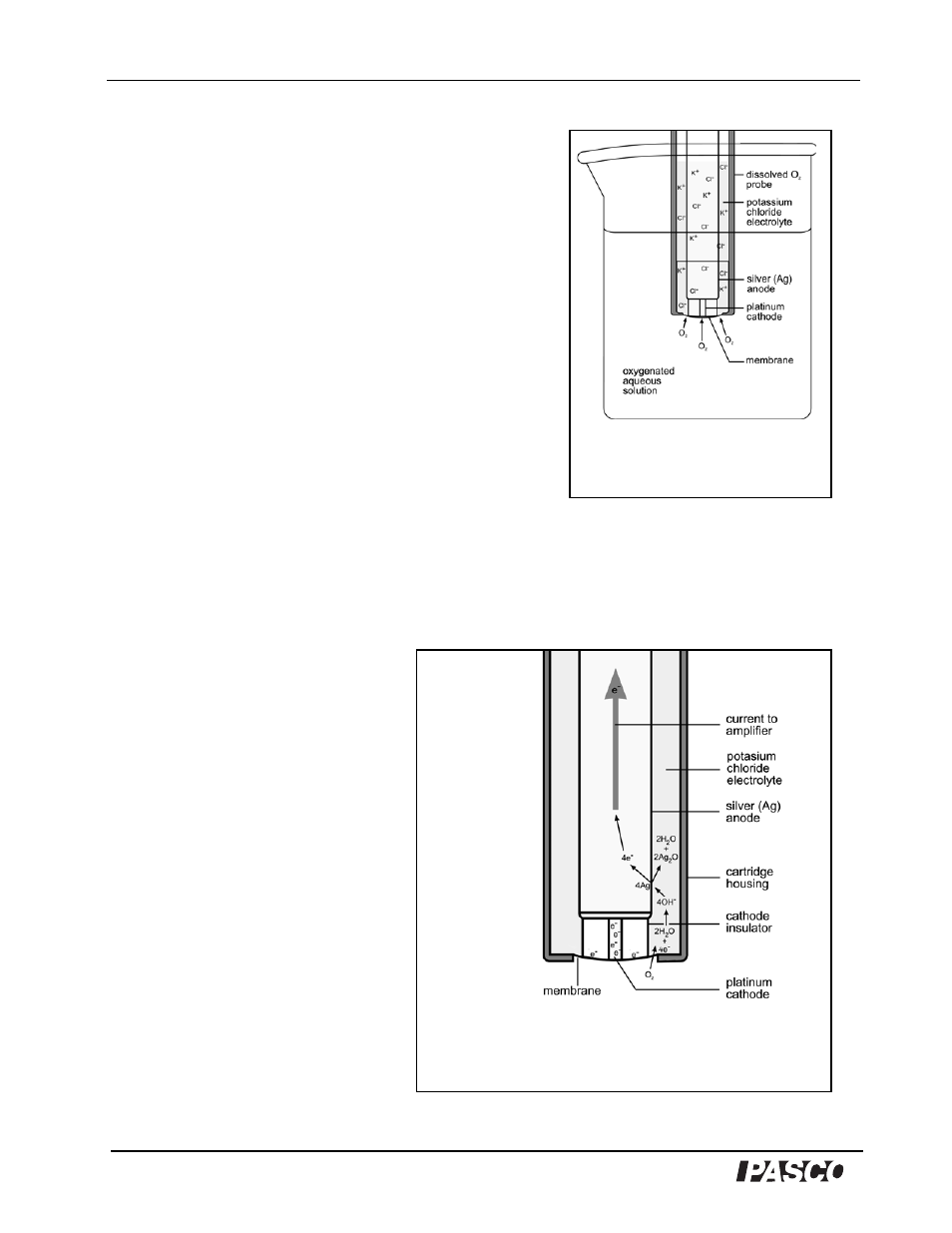
The sensor functions by measuring the electric current
produced by a chemical reaction in the probe. The
chemical reaction involves the reduction of oxygen (O
2
)
molecules and the oxidation of the silver (Ag) atoms of
the anode. A voltage of 0.7 volts is applied across the
electrodes, causing the desired redox reaction (see
below) to be favored. When the dissolved O
2
probe is
placed in an aqueous medium, such as deionized water
that contains dissolved O
2
, the dissolved O
2
molecules
diffuse across a thin silicon membrane into the
electrolyte that surrounds the electrodes of the probe
(Figure 1). The membrane is semipermeable, allowing
the dissolved O
2
to pass through it, but preventing
passage by most other molecules that might interfere
with the chemical reactions at the electrodes. The
chemical reactions produce electrons that cause electric
current to flow through the sensor's electric circuit. Since
the rate of diffusion is dependent on the concentration of the dissolved O
2
, the number of
diffused O
2
molecules will vary approximately in direct proportion to the concentration of
dissolved O
2
in the test solution. Accordingly, the number of electrons produced by the redox
chemical reactions of the dissolved O
2
will be almost directly proportional to the
concentration of dissolved O
2
in the test solution.
The following is an overview of the
chemical and electrical processes at
each of the electrodes that are
involved in measuring dissolved O
2
with the Dissolved Oxygen Sensor.
1
As soon as the dissolved O
2
molecules pass through the silicon
membrane into the electrolyte
solution, they come into close
proximity to the platinum cathode
(Figure 2). The negative charge
(excess electrons) of the cathode
induces the reduction of the
dissolved O
2
, forming hydroxide
ions (OH
-
):
Figure 1
Oxygen molecules pass through the
semipermeable membrane into the
electrolyte surrounding the electrodes
Figure 2
O
2
molecules diffuse across the membrane and react with water
molecules in the presence of electrons from the cathode to form
hydroxide ions (OH
-
). Hydroxide ions diffuse to the anode and react with
silver (Ag) atoms, forming silver oxide (Ag
2
O), water, and free electrons.
O
2
2H
2
O
4e
-
4OH
-
Reduction potential = 0.40V
+
+
