Theory of dissolved oxygen – PASCO PS-2108 Dissolved Oxygen Sensor User Manual
Page 14
PASPort Dissolved Oxygen Sensor
Model No. PS-2108
10
®
Theory of Dissolved Oxygen:
What is the chemical mechanism by which diatomic oxygen dissolves in water?
This is a particularly eloquent description of the mechanism of oxygen solubility in water:
[from Water on the Web
1
]
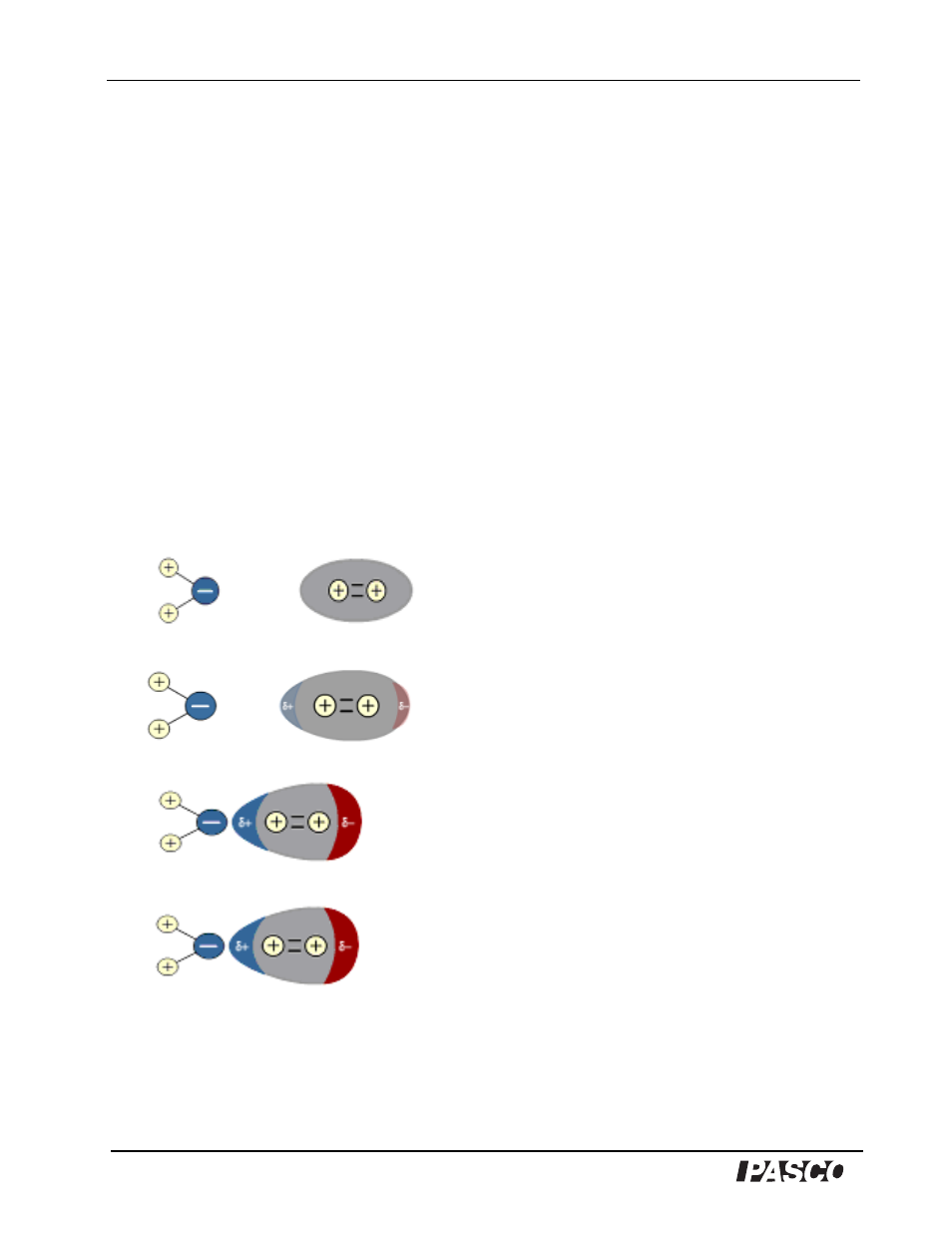
Water, as a polar molecule, induces an accumulation of electron density (dipole moment) at one end of nonpolar
gas molecules such as oxygen (O
2
) and carbon dioxide (CO
2
). In animation, observe a polar water molecule
approaching a nonpolar O
2
molecule. The electron cloud of O
2
is normally distributed symmetrically between
the bonded O
2
atoms. As the negative end of the H
2
O molecule approaches the oxygen molecule, the electron
cloud of the O
2
moves away to reduce the negative-to-negative repulsion. As a result, a dipole (a molecule with
positive and negative charges separated by a distance) has been induced in the nonpolar O
2
molecule, causing O
2
and H
2
O to become weakly attracted to each other. This intermolecular attraction between the oppositely charged
poles of nearby molecules is termed a dipole- induced dipole force. The creation of these forces then explains the
mechanism by which gases dissolve in water.
Still images from the Water on the Web animation:
II. What factors influence the amount of dissolved oxygen in water? There are several
factors that can influence the amount of gaseous diatomic oxygen (as well as other gases) that
will dissolve in water. When speaking of gases dissolving in water, discussion is limited to
gases that do not chemically react with the water.
Induced Dipoles
The electron cloud of an isolated Oxygen
molecule is distributed symmetrically
between the bonded O atoms.
As the negative end of the polar water
molecule approaches…
…the O
2
electron cloud is moved away to
reduce repulsion between the O
2
cloud and
the negative end of the water molecule.
The O
2
molecule itself becomes polar.
A dipole has been induced in the otherwise
nonpolar O
2
molecule. The H
2
O and the O
2
are now weakly attracted to one another.
