Calculations – Parr Instrument 6755 User Manual
Page 27
Calculations
6755
5
w w w . p a r r i n s t . c o m
25
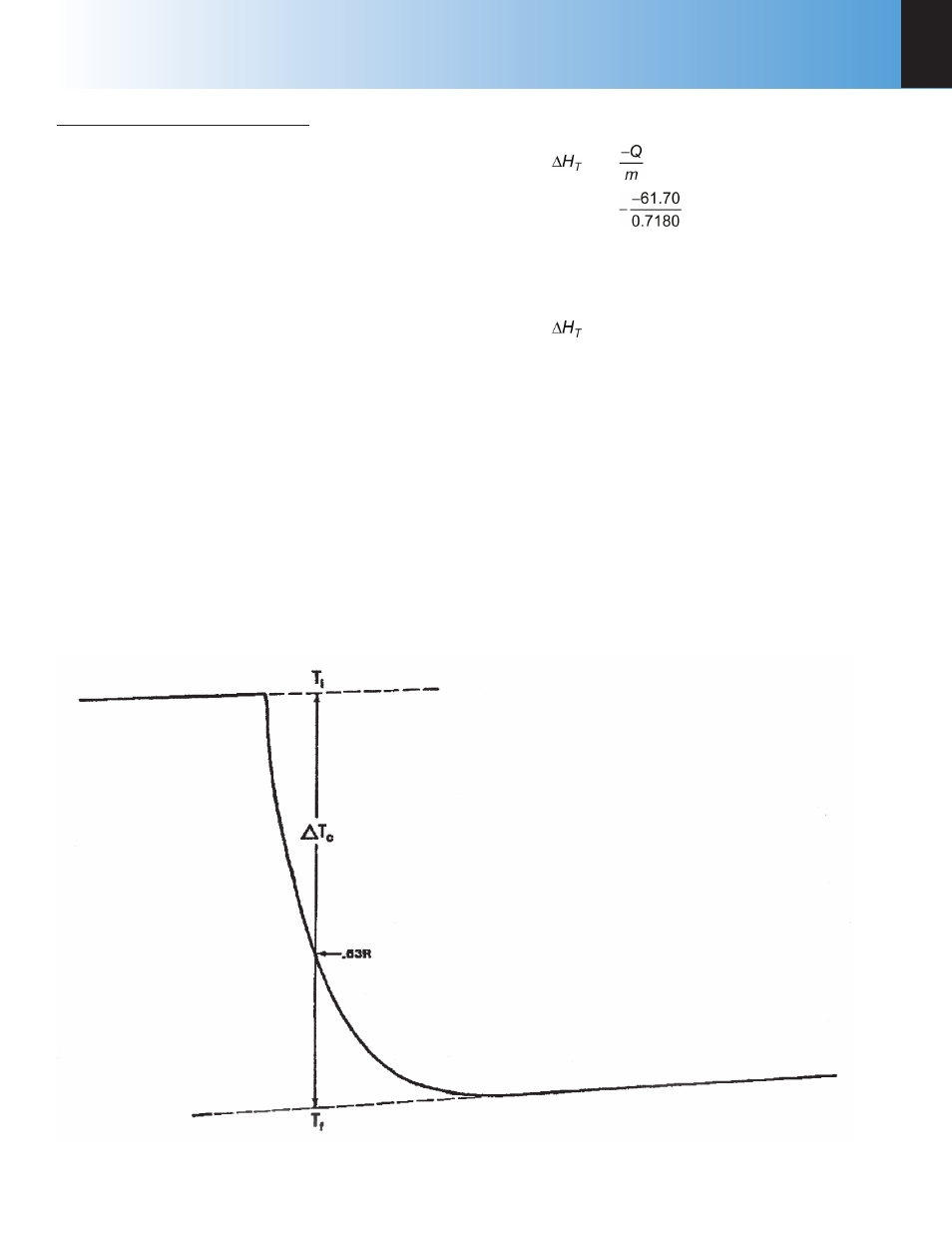
Figure 4
Endothermic Reaction
Example - Endothermic Reaction
Problem: Determine the heat of solution of solid
potassium nitrate, KNO
3,
when dissolved in
water.
KNO
3
m
= 0.7180 gram
Distilled water = 100.00 grams
Corrected temperature rise
∆T
C
=
-0.508 °C (from Figure 3)
T(0.63R)
=
25.400 °C
Energy equivalent
e
= 121.46 cal/°C
Energy evolved
Q
= (∆T
C
) (e)
= (-0.508) (121.46)
= -61.70 calories
Enthalpy change
=
=
= -34.99 cal/g @ 24.885 °C
Or, multiplying by the molecular weight of KNO3
= (85.94) (101.10)
= 8.69 Kcal/mole @ 25.400 °C
0.7180g solid KNO
3
dissolved in
100.00g of distilled water
Temperature at
time of mixing
25.714 °C
R = ∆T
C
-0.508 °C
0.63R
-0.320 °C
T
i
25.720 °C
T(0.63R) = T
i
+ 0.63R
25.400 °C
T
f
= T
i
+ R
25.212 °C
- 1341 (16 pages)
- 1108 (20 pages)
- 1901 (2 pages)
- 1104 (12 pages)
- 1121 (4 pages)
- 1755 (2 pages)
- 1552 (1 page)
- DP8340R Series (52 pages)
- Attaching Platinum Fuse Wire (1 page)
- 1281 (2 pages)
- 1271 (2 pages)
- Performa Therm Liquid Calorimetric Thermometers (2 pages)
- 1108P (20 pages)
- 1356 (41 pages)
- 6300 (130 pages)
- 6200 (94 pages)
- 6200 (88 pages)
- 6100 (82 pages)
- 6510 (16 pages)
- 1564 (12 pages)
- 6400 (103 pages)
- 6400 (110 pages)
- 6772 (70 pages)
- 6725 (76 pages)
- 6750 (36 pages)
- 1108V (4 pages)
- 1108R (20 pages)
- Safety Rupture Disc Assemblies (8 pages)
- Magnetic Drive (16 pages)
- 44HC5 Metal Gaskets (1 page)
- Flexible Graphite Gaskets (1 page)
- Pressure Relief Valves (2 pages)
- Series 5100 (32 pages)
- Series 4520 (32 pages)
- Series 4530 (28 pages)
- Series 4540 (32 pages)
- Series 4550 (28 pages)
- Series 4560 (28 pages)
- Series 4570 (28 pages)
- Series 4580 (28 pages)
- Series 4590 (28 pages)
- Series 5000 (20 pages)
- Series 4555 (48 pages)
- 4575/76 HP/HT (24 pages)
