2 evaporation of the solvent, 3 detection – BUCHI ELS Detector C-650 User Manual
Page 16
C-650
Operation Manual, Version B
4 Description of function
16
4.2.2
Evaporation of the solvent
A heated tube is used to evaporate the solvent. The exit of the heated tube leads directly into the
detection chamber.
In liquid chromatography, water and organic solvents with low boiling points are typically em-
ployed (e.g. CH
3
OH, CHCl
3
, CH
3
CN). A typical mobile phase for a reverse phase separation us-
ing Evaporative Light-Scattering Detection might be CH
3
OH/H
2
O (60/40) while a typical mobile
phase for normal phase separation might be C
6
H
14
/CHCl
3
(60/40).
If acids, bases and salts are used to modify mobile phase to provide the desired separation, they
should be able to be readily evaporated, sublimed or decomposed into gases in the evaporation
tube. Mobile phase modifiers that are commonly used when an Evaporative Light-Scattering
Detector is employed include NH
4
OH, (C
2
H
5
)
3
N, NH
4
Acetate, NH
4
Formate, HCOOH, CH
3
COOH
and CF
3
COOH.
4.2.3
Detection
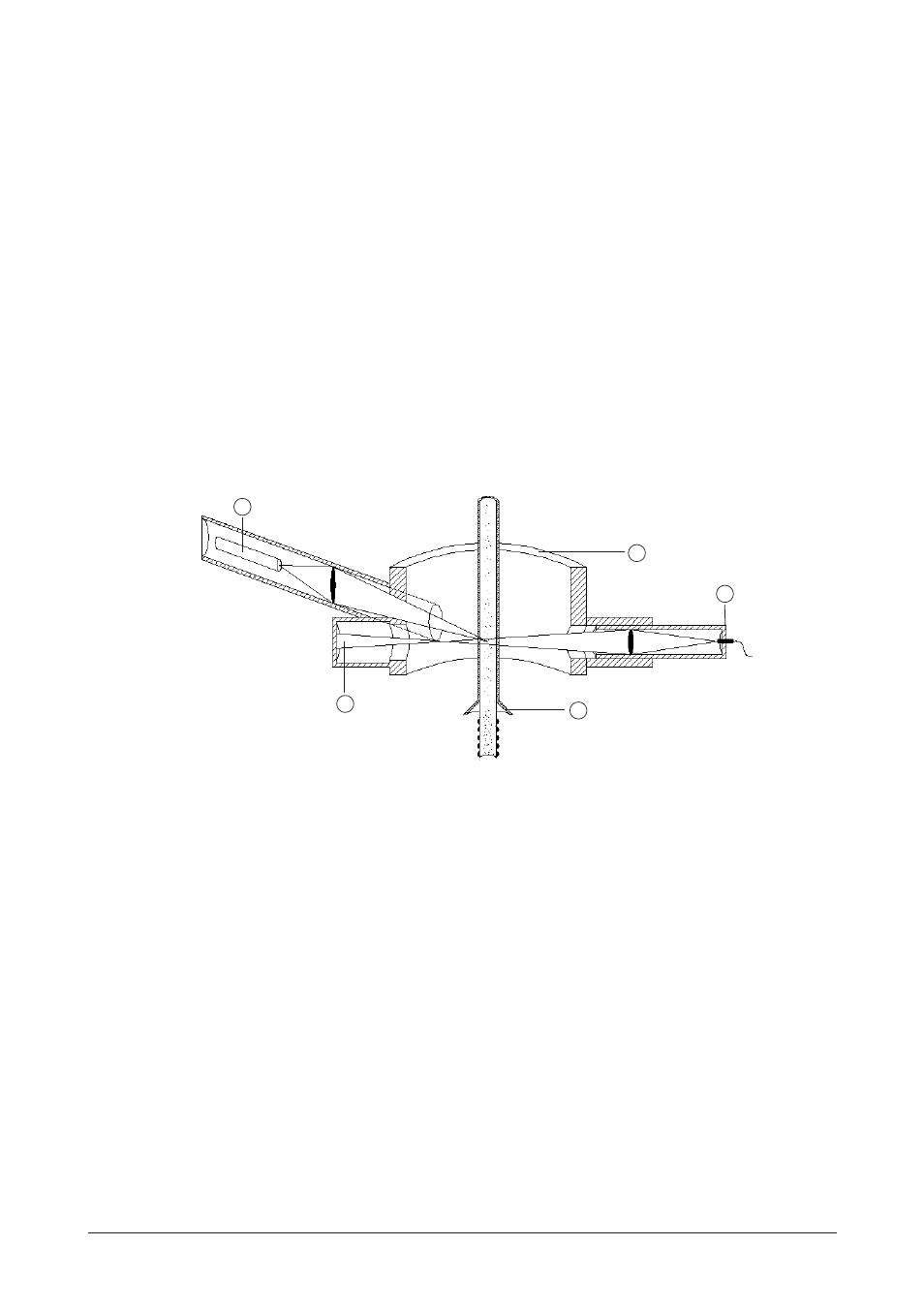
The carrier gas transports the microparticles from the heating tube into the detection chamber
(Fig 4.5).
1
2
3
4
5
a Photomultiplier
b Detection chamber
c LED
d Additional gas
e Light trap
Fig 4.5: Detection chamber
The detector chamber contains a Light Emitting Diode (LED) and a photomultiplier that is posi-
tioned at an angle of 100° with respect to the light beam (Fig 4.5). When the carrier gas contains
microparticles, the light is scattered and is detected by the off-axis photomultiplier.
The intensity of the scattered light is a function of the mass of the scattering particles and gener-
ally follows an exponential relationship, which is shown in the following equation.
I = k m
b
where:
I is the intensity of light
m is the mass of the scattering particles
k and b are constants
