Bio-Rad Sequi-Gen GT Sequencing Cell User Manual
Page 15
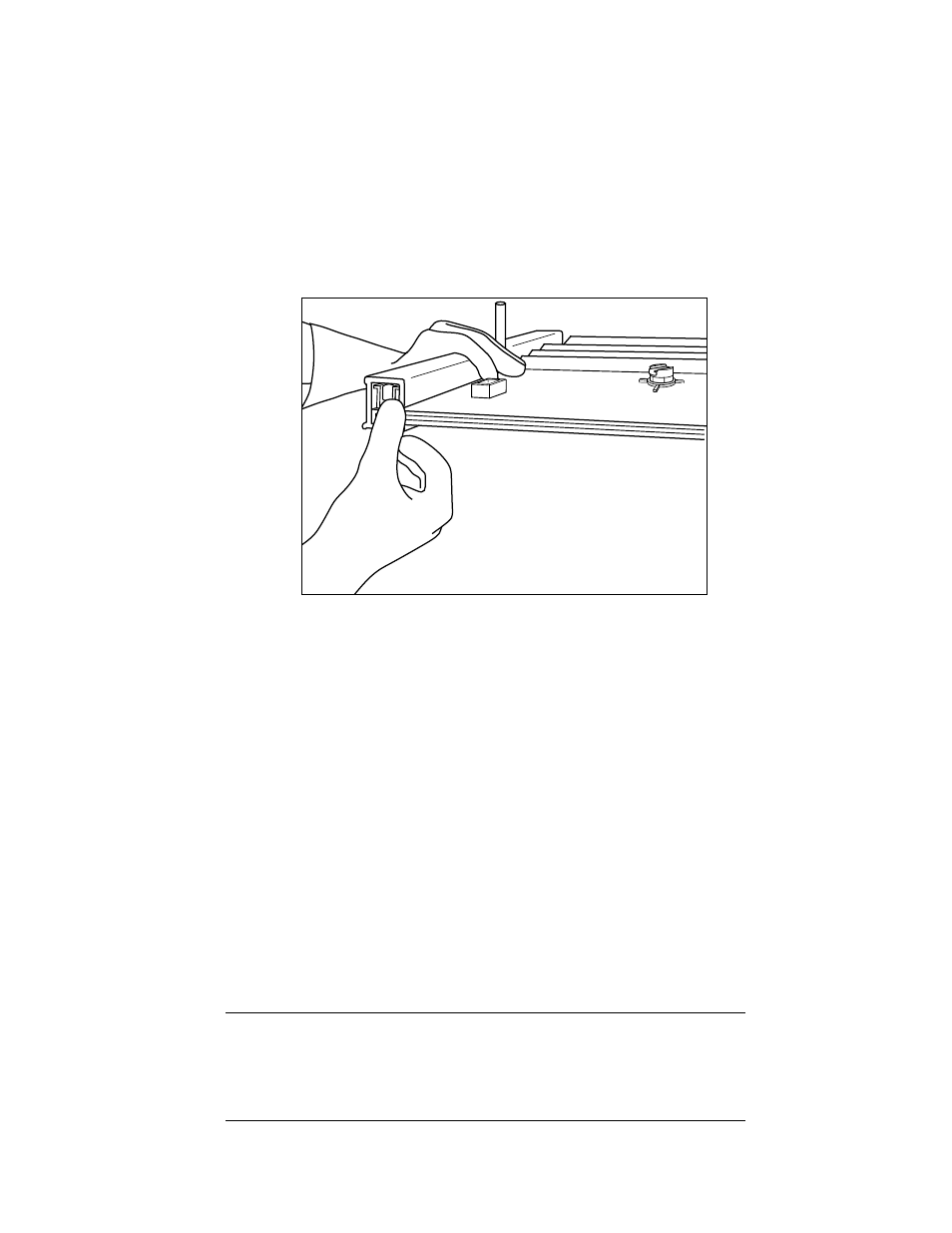
5. Lay the IPC assembly on the benchtop with the IPC panel (drain port side) facing up.
• Check the alignment of the glass plates, spacers and clamps. The bottom of the glass
plates, spacers and clamps should be flush. If either glass plate, spacer, or clamp is
not properly aligned or flush, adjust the alignment by loosening the clamps and move
clamps, glass plates and spacers into alignment (Figure 4.3).
• Tighten the clamps by moving the levers back down towards the IPC after the assembly
is flush.
Fig. 4.3. Alignment of glass plate sandwich.
6. To avoid incompatibility problems between combs and spacers after the gel is cast, check the
fit of the combs in the assembled Sequi-Gen GT cell by trying to place them between the plates.
• If the combs clearly will not fit between the plates without damaging the comb, try a
different comb. Optimally, combs should demonstrate slight resistance to being placed
between the glass plates.
4.3 Casting the Gel
Section 7.1 contains a checklist of required items for DNA sequencing. Polyacrylamide
is a hazardous chemical and neurotoxin. Always wear gloves, lab coat, and safety glasses
while working with polyacrylamide.
1. Prepare the gel solution described in Section 7.1 and 7.2.
• Degas the gel solution for 5-15 minutes under a strong vacuum (
≥
26 in./Hg) to insure
reproducible gel porosity.
Table 4.2 Required Gel Volumes Using the Precision Caster Assembly
IPC
0.25 mm
0.4 mm
0.75 mm
0.25 – 0.75 mm 0.40 – 1.2 mm
Size
spacers
spacers
spacers
wedge spacer
wedge spacer
21 x 40 cm
25 ml
35 ml
70 ml
50 ml
60 ml
21 x 50 cm
30 ml
45 ml
90 ml
65 ml
85 ml
38 x 30 cm
40 ml
50 ml
90 ml
—
—
38 x 50 cm
55 ml
85 ml
170 ml
120 ml
140 ml
12
