Compression device – Merit Medical Finale Radial Compression Device User Manual
Page 2
Compression Device
Intended Use
The Finale® Compression Device is intended
for compression of access site vasculature after
catheterization procedures.
ContraIndICatIons
The use of the Finale for radial artery compression is
contraindicated in patients with an abnormal Allen’s test
or radial pulse, or insufficient dual arterial supply.
The Finale is not intended for femoral artery
compression.
CaUtIons
Ensure correct placement of the Finale
before removing sheath from access site.
RX Only: Caution: Federal Law (USA) restricts this
device to sale by or on the order of a physician.
Read instructions prior to use.
Product is intended for single use only.
Do not reuse or re-sterilize; do not autoclave.
Do not use if package is opened or damaged.
Contents of unopened, undamaged package are
sterile (via ethylene oxide) and non-pyrogenic.
reUse PreCaUtIon statement
For single patient use only. Do not reuse, reprocess or
resterilize. Reuse, reprocessing or resterilization may
compromise the structural integrity of the device and/
or lead to device failure which, in turn, may result in
patient injury, illness or death. Reuse, reprocessing or
resterilization may also create a risk of contamination
of the device and/or cause patient infection or cross-
infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the device may lead to injury, illness or
death of the patient.
The Finale should be used by clinicians with adequate
training in the use of the device.
WarnIngs
• Patient should not be left unattended while
the
Finale is in place
• Ensure correct alignment of the Finale
Compression Dial prior to use
• Do not over tighten device
• Do not leave Finale on for inappropriately
long periods of time as tissue damage may occur
• Arterial pulse distal to the compression plate should
be monitored to ensure the artery is not completely
occluded as arterial damage and/or thrombosis
could occur
• When reducing compression, be sure to grasp dial
while pushing in Release Tabs 1 and 2
PotentIal ComPlICatIons
Potential complications include, but are not limited
to: arterial occlusion, hematoma, hemorrhage, pain,
numbness, nerve or tissue damage, and risks normally
associated with percutaneous diagnostic and/or
interventional procedures. Patient must be monitored
for hemostasis; compression pressure should be
adjusted according to facility protocol.
ProdUCt desCrIPtIon
(1) Finale Compression device
(1) label for compression time
InstrUCtIons For Use
The following instructions provide technical direction
but do not obviate the necessity of formal training in
the use of the Finale. The techniques and procedures
described do not represent all medically acceptable
protocols, nor are they intended as a substitute for the
clinician’s experience and judgment in treating any
specific patient.
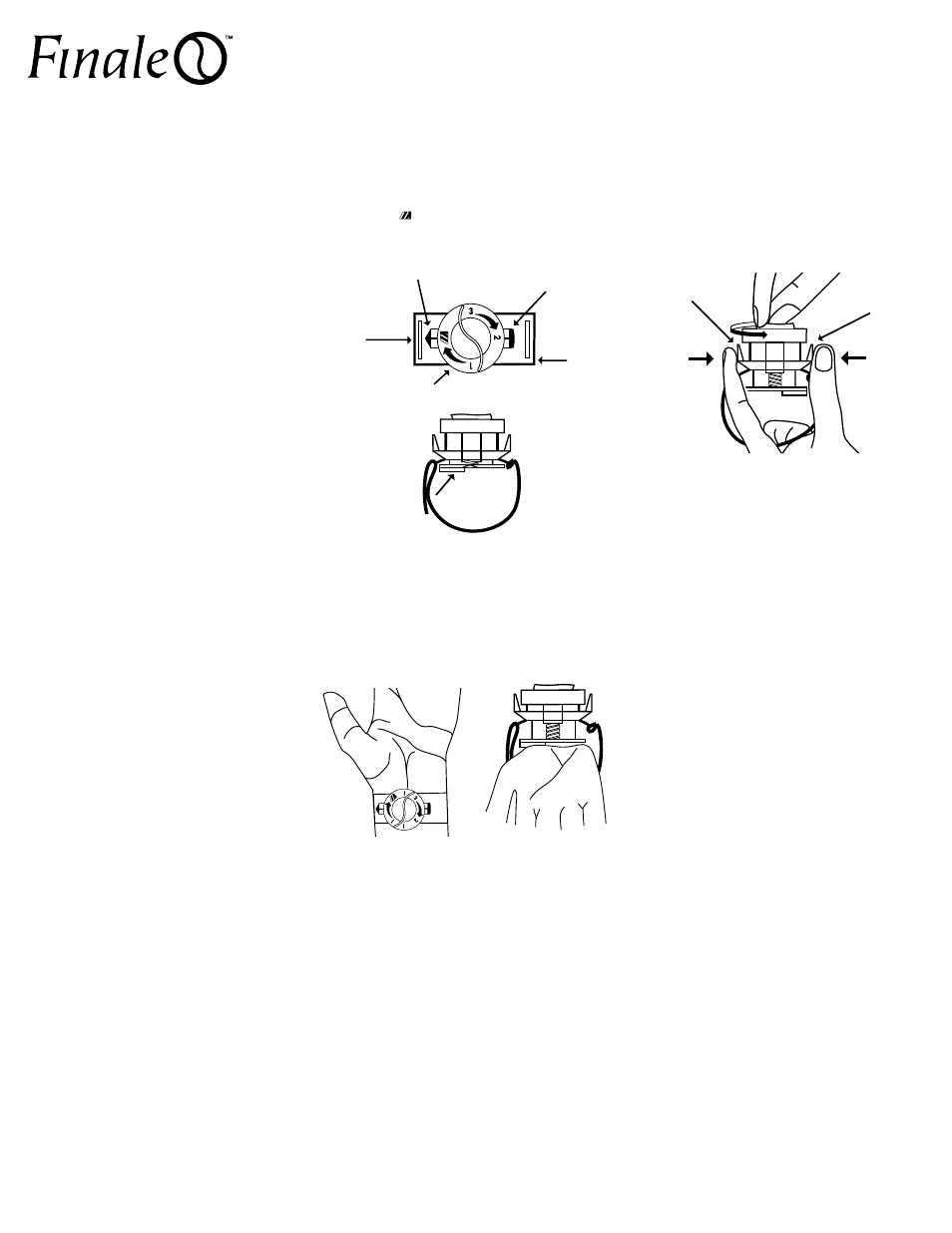
1. Remove the sterile Finale from the package.
2. Simultaneously depress Release Tabs 1 and 2 while
rotating the compression dial counter-clockwise
until the is aligned with Release Tab 1. This
ensures correct alignment of the compression
dial and plate prior to placement.
Warning: ensure correct alignment of the
compression dial prior to use.
3. Place device over access site where compression is
desired. Loop the VELCRO® brand strap through the
slot; attach and tighten appropriately. Do not over
tighten strap.
note – The Finale Compression Device may be used on
the right or left arm.
Caution: Ensure correct placement before removing
sheath from access site.
4. While slowly removing sheath, rotate compression
dial clockwise to achieve hemostatis.
note - The numbers on the compression dial are for
convenience only.
note - The amount of compression necessary to
achieve hemostasis can vary from patient to patient.
Warning – do not over tighten device.
5. Check for bleeding at access site; if there is bleeding,
adjust the compression dial by turning dial clockwise.
note – Use minimum amount of pressure necessary to
achieve hemostasis.
6. Initial compression should be maintained for
approximately 30 minutes. The compression device
should be loosened every 15-30 minutes until
hemostasis is achieved.
to decrease compression:
Warning - Be sure to grasp dial while pushing
release tabs 1 and 2
7. Hold the compression dial with one hand, and
squeeze Release Tabs 1 and 2 with the other hand.
While squeezing Release Tabs, rotate the dial
counter-clockwise to desired compression.
Release tabs and note the compression level
and time on label or chart.
8. Once hemostasis is achieved, remove compression
device and discard.
Warning - Patient should not be left unattended
while device is in place.
9. Post-procedure care should follow standard
facility protocol.
Compression
Plate
Tab 2
Tab 1
Release Tab 2
Release Tab 1
Release Tab 2
Securement Band
Compression Dial
Slots for Band
