Ocean Optics CHEM2000 User Manual
Page 29
3: Experiment Tutorial
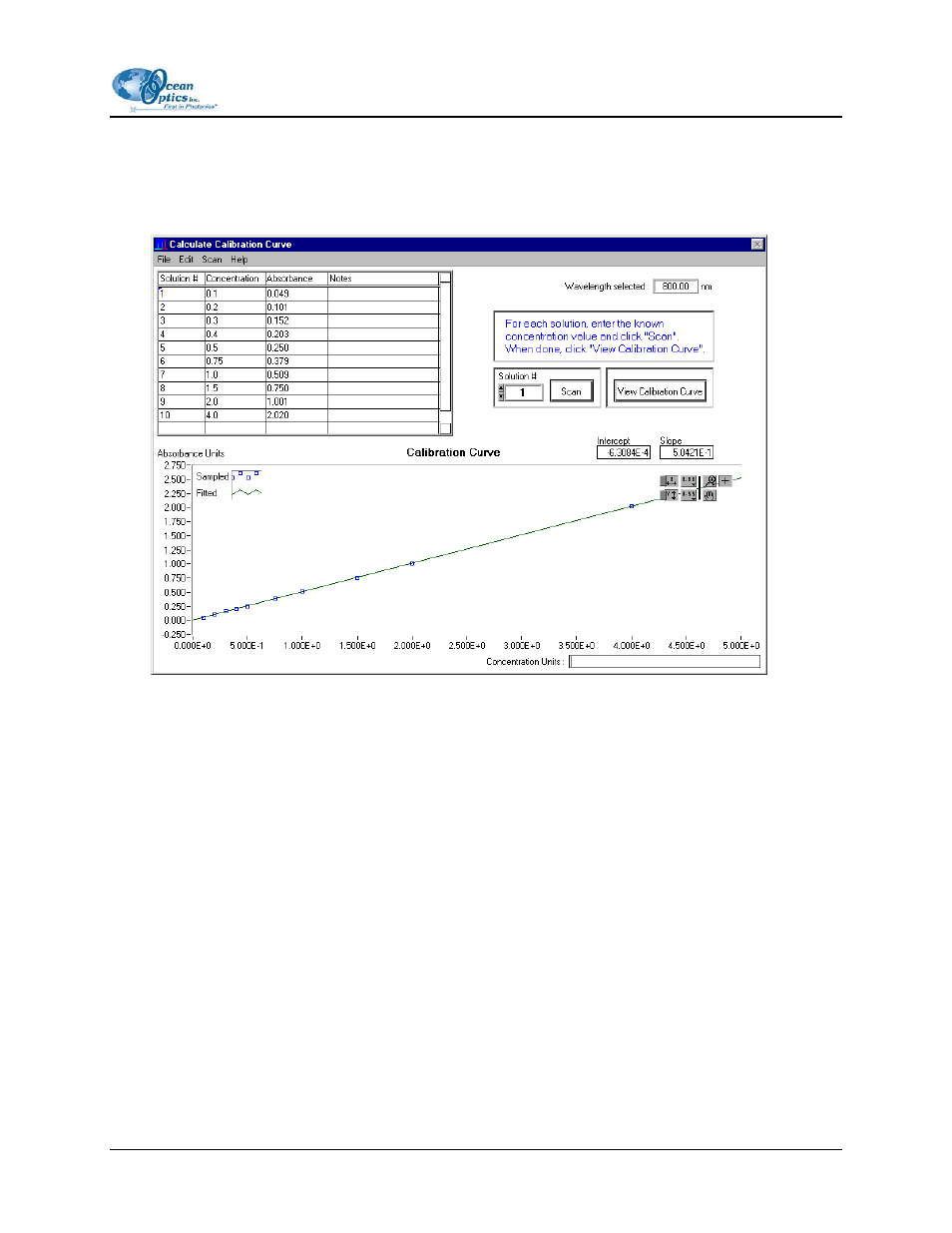
8. Click
the
Scan button or select Scan | Solution from the menu. The absorbance value will appear
next to the concentration for Solution #1. At any point, you can select Edit | Clear from the menu
to clear the dialog box of all data.
9. Take Solution #1 out of the cuvette holder and put in another standard solution with the next
highest known concentration. Enter the known concentration of the standard solution in the chart
in the Concentration column, next to Solution #2.
10. Click the Scan button or select Scan | Solution from the menu. The absorbance value will appear
next to the concentration for Solution #2.
11. You may continue to scan solutions with known concentrations. You must scan at least 2 in order
to achieve a calibration curve.
12. When you have completed taking scans of your solutions with known concentrations, click the
View Calibration Curve button. You will then have the Intercept and Slope of your curve. The
Slope is the
ε necessary to compute Beer’s Law and to find the unknown concentration of a
solution.
13. At this time, you may also select a label for your concentration values, such at Moles per Liter,
in the Concentration Units box. This is only a label and does not affect the data in any way.
105-00000-CHM-02-0405
PRELIMINARY DRAFT
23
