Safety, Safety standards, Ce marking – Philips 862478 User Manual
Page 3: Europe
iii
Safety
All warnings, precautions and notes are located in the chapters that follow. You must read all
of this safety information before you begin monitoring with your C3 patient monitor.
Safety
Standards
The C3 Patient Monitor is compliant with the following safety standards:
•
UL 2601-1
•
CAN/CSA C22.2 No. 601.1-M90
•
EN60601-1
•
EN60601-2-27
•
EN60601-2-30
•
EN60601-2-49
•
EN865
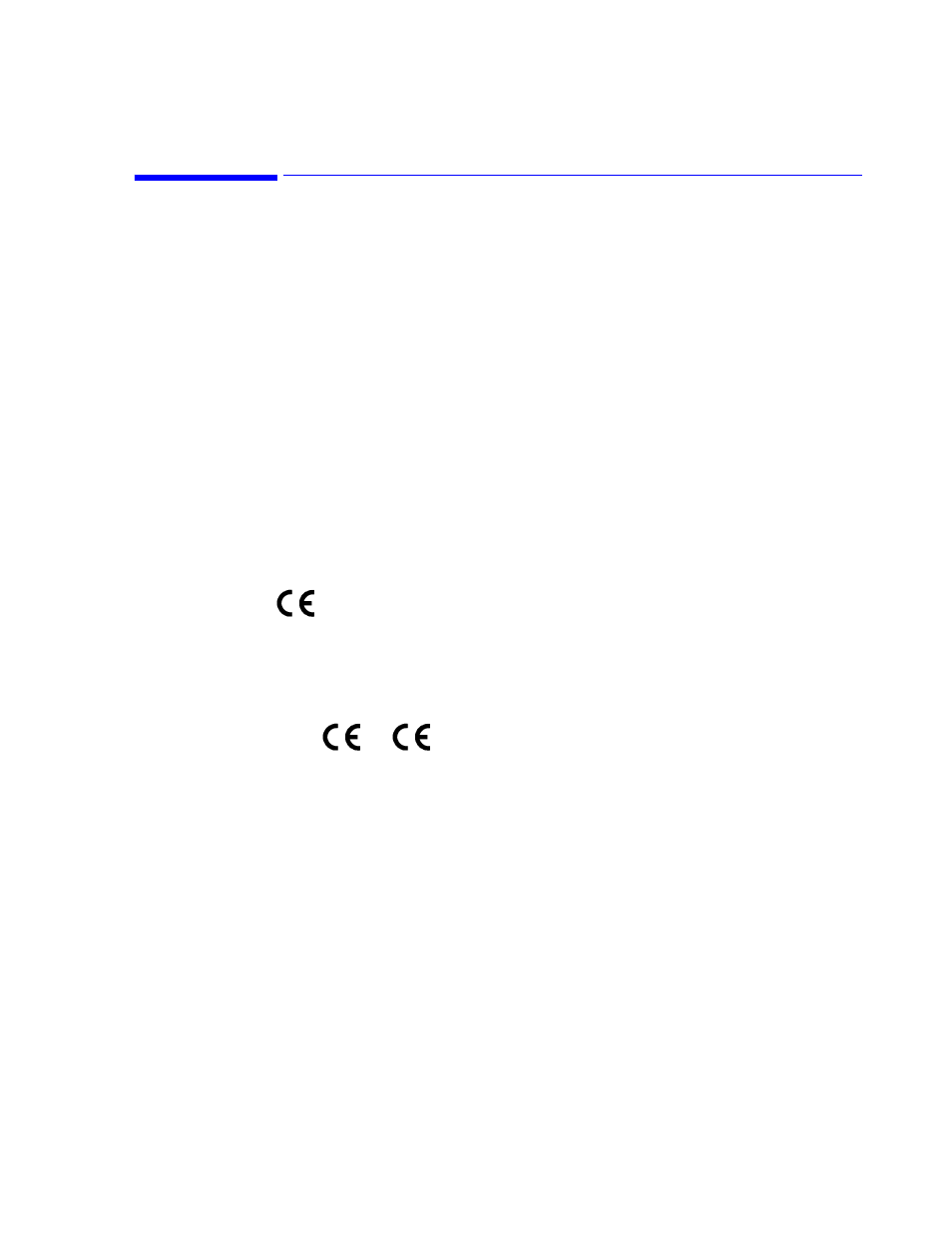
CE Marking
Europe
The following products and accessories from Philips Medical Systems carry the CE mark
to Council Directive 93/42/EEC.
C3 Products: - 862474
- 862478
Accessories: - 21075A
- 21076A
- 21078A
- 21090A
- M1837A
- M4820A
- M4818A
The following accessories from Philips Medical Systems carry one of the following CE
marks:
or
to Council Directive 93/42/EEC.
Accessories: - M1191A
- M1192A
- M1194A
- M1941A
- 40493D
- 40493E
- M1980A
- M1981A
- M1510A
- M1550C
- M1611A
- M1613A
- M1615A
- M1879A
- M1878A
- M1877A
- M1876A
- M1875A
- M1874A
- M1576A
- M1575A
- M1574A
- M1573A
- M1572A
- M1571A
- M1577A
- M1578A
- M1579A
- 40401E
- 40401D
- 40401C
- 40401B
- 40401A
- M1598B
- M1599B
- M2526A
- M2525A
- M2524A
- M2528A
- M2522A
- M2521A
- M2520A
- M1920A
- M1922A
- M1921A
- M4816A
- M4817A
Accessories from companies other than Philips Medical Systems carry CE markings
appropriate to the accessory.
Additional accessories not identified above fall outside the definition of a medical device.
The Former Agilent Technologies’ Healthcare Solutions Group is now a part of Philips
Medical Systems. Some accessories may still be branded with the Agilent name.
0123
0366
