Sievers membrane conductometric toc technology, Dataplus software, Firmware upgrades – GE P&W Ultrapure and Drinking Water TOC Analyzers - Sievers 860 Laboratory TOC Analyzer & GE Autosampler RT12 User Manual
Page 3: Options and accessories
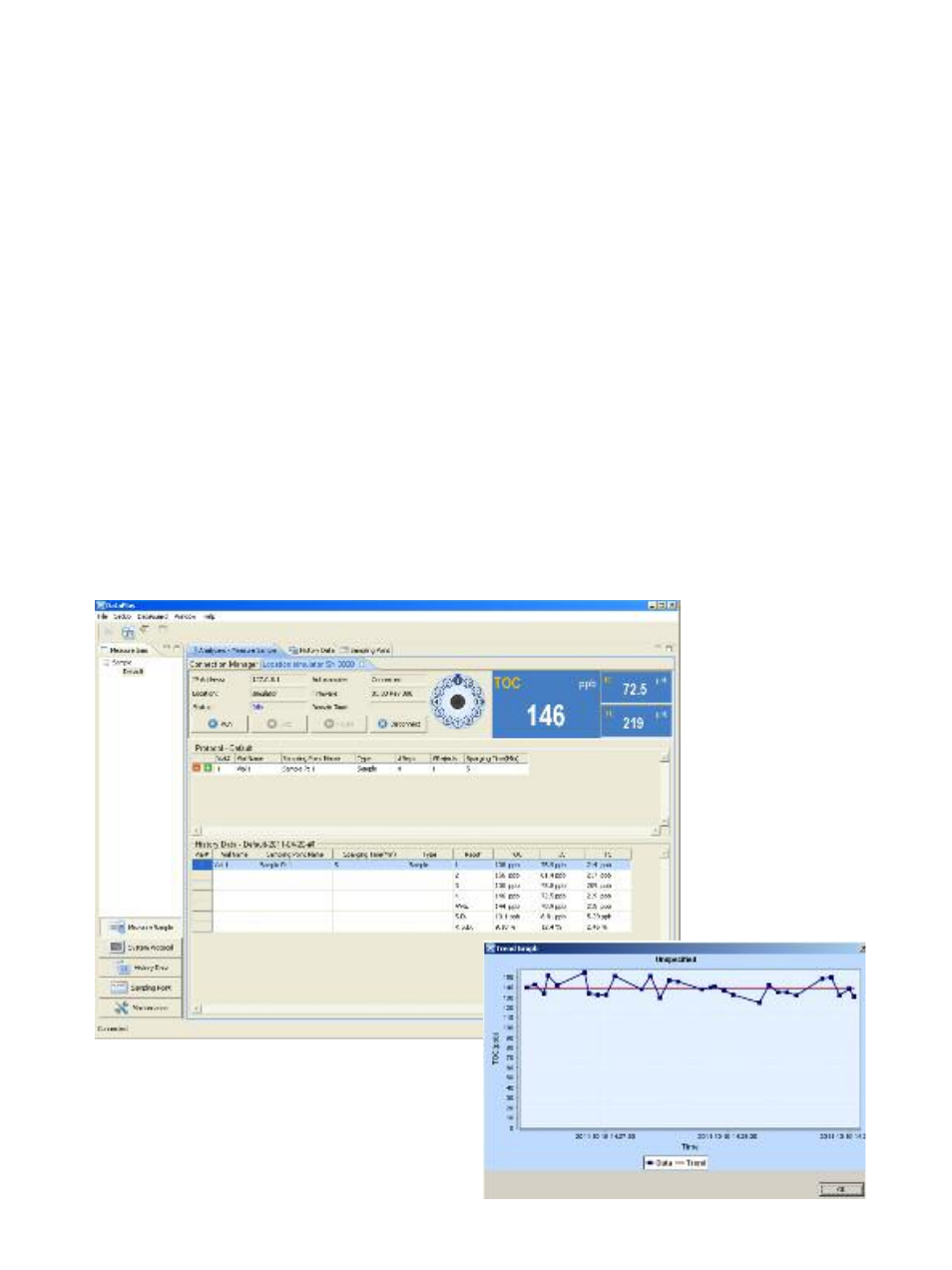
DataPlus Measurement Screen
DataPlus Sample Point
Trending Graph
Sievers Membrane Conductometric
TOC Technology
The Sievers 860 uses the Sievers Membrane
Conductometric Detection method, which has proven to
be an extremely reliable technique for measuring TOC. It
uses a gas-permeable membrane that selectively pass-
es only the CO
2
produced from the oxidation of organics.
By preventing compounds such as acids, bases, and
halogenated compounds from interfering with the mea-
surement of CO
2
from oxidation, the Membrane
Conductometric Method delivers unmatched sensitivity,
selectivity, stability, accuracy, and precision. This tech-
nique also can tolerate higher conductivity and remove
inorganic carbon more effectively than TOC analyzers
that use the direct conductometric technology.
DataPlus Software
The Sievers 860 can operate standalone or with a PC.
When using a PC, the innovative, easy-to-use
DataPlus* software allows users to operate the Sievers
860, manage and analyze water sample data, and
comply with pharmaceutical water testing regulations.
The most robust laboratory TOC data management
tool available, DataPlus lets you:
• Set up sampling protocols in just minutes.
• Create trending charts for a single sample point for
internal quality control and troubleshooting.
• Export data as .csv or .xls files, and print reports and
trending charts in pdf format.
• Track consumables usage and conduct routine
maintenance easily.
• Run up to four Sievers TOC analyzers from one PC via
an Ethernet connection.
Firmware Upgrades
Firmware can be updated easily using a USB key. You
can take advantage of the latest features and optimize
performance without changing hardware.
Options and Accessories
DataGuard Software
Sievers DataGuard software for the Sievers 860 offers
comprehensive support for compliance with the FDA’s
requirements for control of electronic records and elec-
tronic signature. DataGuard provides an administra-
tively controlled user access system, complete audit
trail functionality, and electronic signature manage-
ment for compliance with 21 CFR Part 11.
