Approval, Event log – ChemoMetec NC-3000 User Manual
Page 51
991-3003 Rev. 1.0
August 2013
51
3000™ application, and are restricted in their use of the application. Running the NucleoView NC-3000™
application as one of those users makes it impossible to break the 21 CFR Part 11 compliance.
Approval
All results can be approved by a user in a user group that is allowed to approve data.
The group right to approve data can be setup in the Tools->Options menu in the tab 21 CFR Part 11. Also it
is possible to setup whether a user should be allowed to self approve.
Event Log
The event logs created by the NucleoView NC-3000™ application are always stored in the current results
folder. Changing this folder will create a new event log file in the newly set results folder.
It is recommended to backup the event logs, archived and aged according to the same rules as e.g. the
results created by the NucleoView NC-3000™ application itself. See
Apart from securing that the user cannot tamper with measured results in the NucleoView NC-3000™
application in 21 CFR Part 11 mode, all operations changing the behavior of the NucleoView NC-3000™
application, all new files created etc. are logged in the event log system. The user needs to be part of the
NucleoViewAdmin group to see the event log.
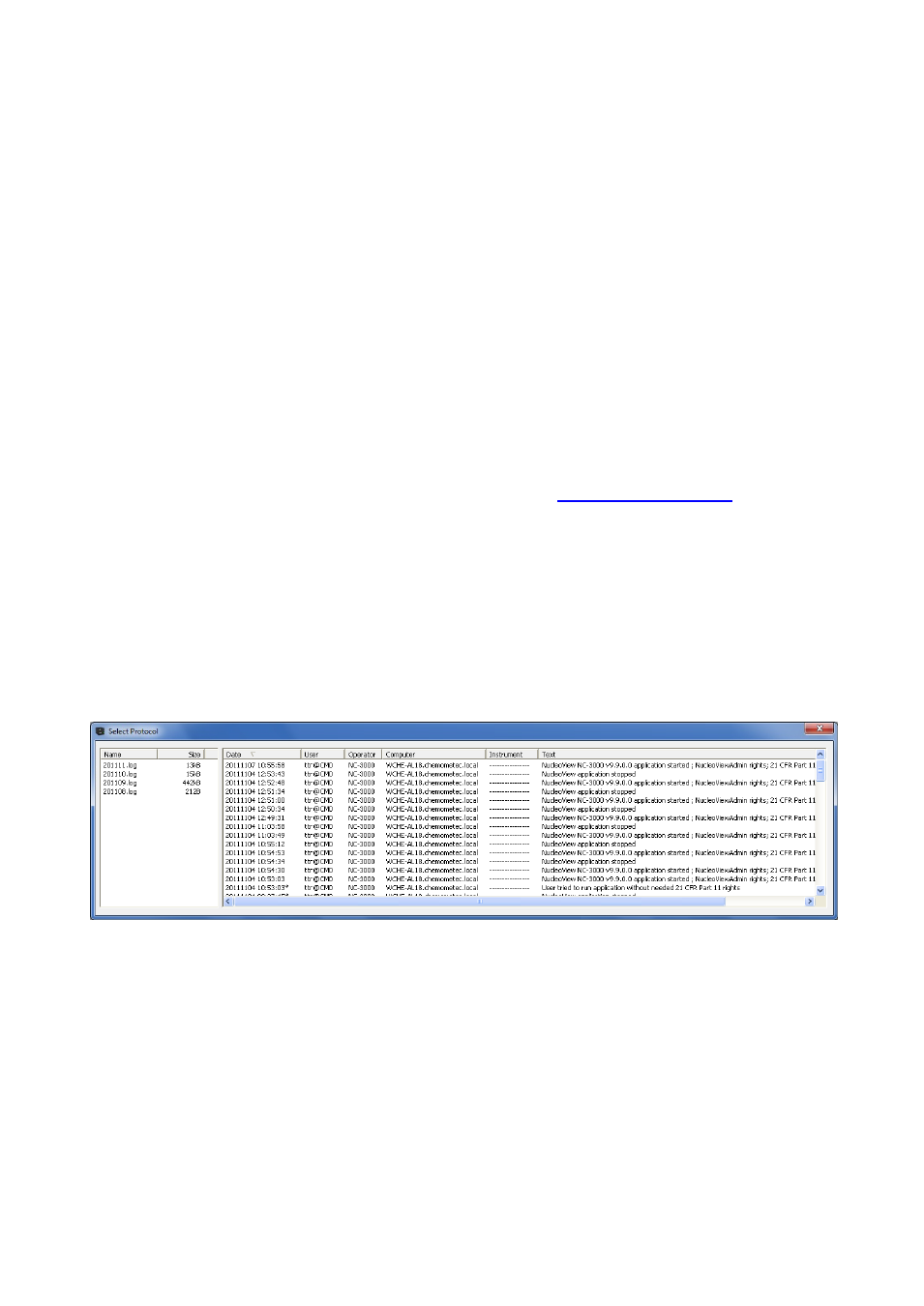
The NucleoViewAdmin user can launch the event log viewer via the main menu View -> Event Logs. This will
bring up the window shown below:
To the left is shown a list of existing event log files, one for each month. Selecting one of these will bring up
the content of this event log file to be shown in the right window. Clicking on one of the headers in the
right window will sort the content according to the selected column either ascending or descending.
Note that in the date column, some of the entries have a small * marked. This indicates that the
NucleoView NC-3000™ application has recorded this event happened when the application was not in 21
CFR Part 11 mode.
