Bio-Rad Nuvia™ IMAC Resin User Manual
Page 14
10 Bio-Scale Mini Nuvia IMAC Ni-Charged
Section 8
Preparing a Cartridge and Subsequent
Purification
Prepare buffer sets for either the native or denaturing purification
protocols using a single buffer set throughout the procedure. To
prepare the cartridge for the procedure, remove the top closure
and connect the cartridge to the chromatography system. Open
the bottom closure and connect the cartridge outlet to the system.
Flush the packing solution (20% ethanol) from the cartridge by
running 2 column volumes (CV) of water at a flow rate of 2 ml/min
(1 ml cartridge) or 10 ml/min (5 ml cartridge). The cartridge is now
ready for the purification steps. Flow rates are given in ml/min and
are specific to the 1 ml cartridge.
If using a 5 ml cartridge for a procedure, substitute the higher flow
rate in the method (refer to the table below).
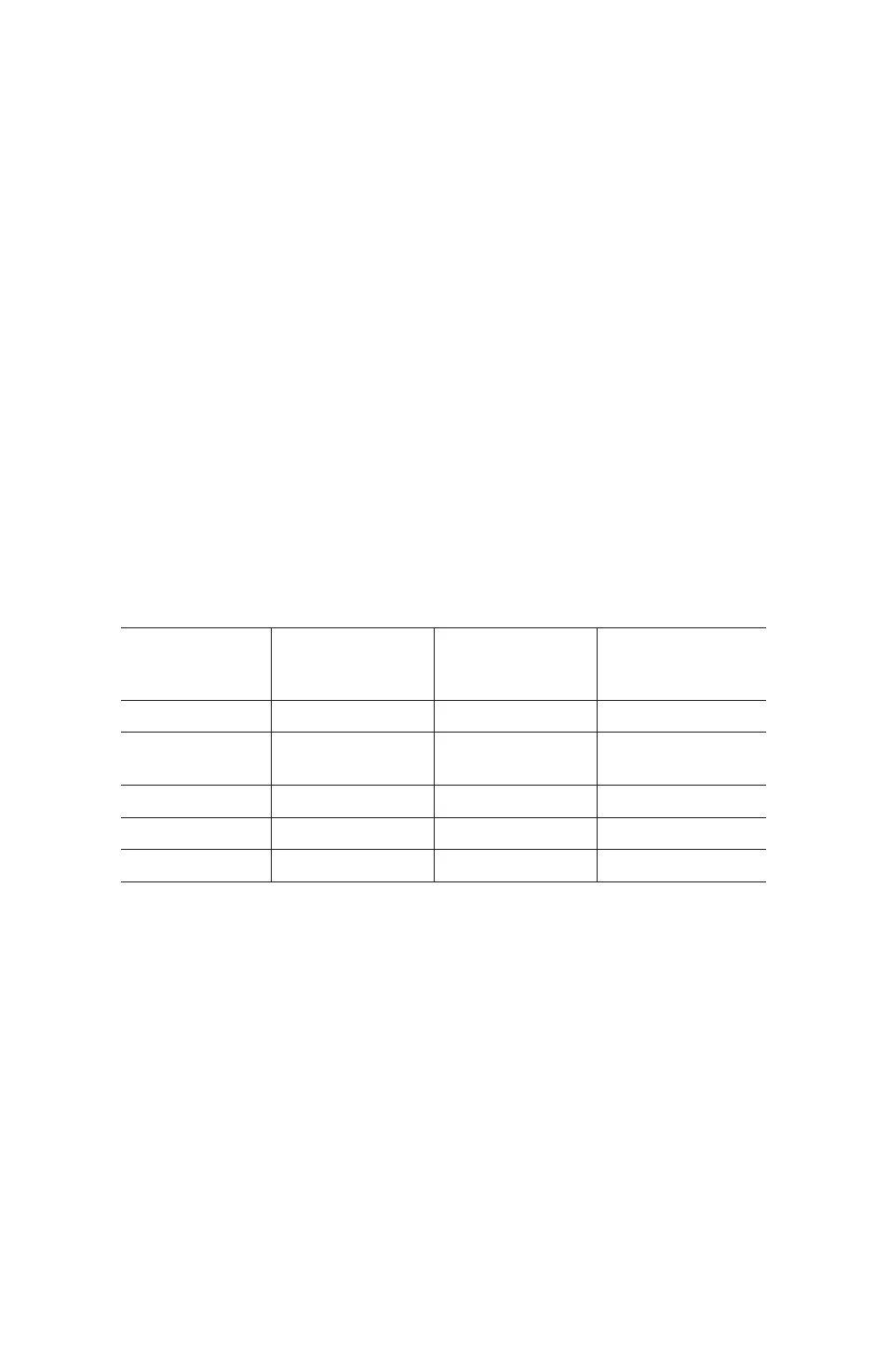
Table 5. Purification method suggestions.
Step
Column
Volumes, CV
1 ml Cartridge
Flow Rate,
ml/min
5 ml Cartridge
Flow Rate,
ml/min
Equilibrate
5
2
10
Lysate load
Varies based on
sample volume
1*
5*
Wash 1
6
1
5*
Wash 2
6
2
10
Elute
5
2
10
* Depending on sample viscosity.
Standard methods that are compatible with any type of
chromatography system are listed in the following steps. To
maximize binding capacity with large proteins (>100 kD), for
purification at 4°C, or for purifications under denaturing conditions,
the lysate load flow rate can be decreased (to 0.5 ml/min for the
1 ml cartridge and 2 ml/min for the 5 ml). Whether this decrease
maximizes flow rate will have to be determined empirically for
individual proteins.
1. Equilibrate the cartridge with 5 CV of equilibration/wash
buffer 1 at 2 ml/min.
