4 3 )] ( [ mbar hg mm – Heidolph LABOROTA 4000 eco User Manual
Page 62
62
E
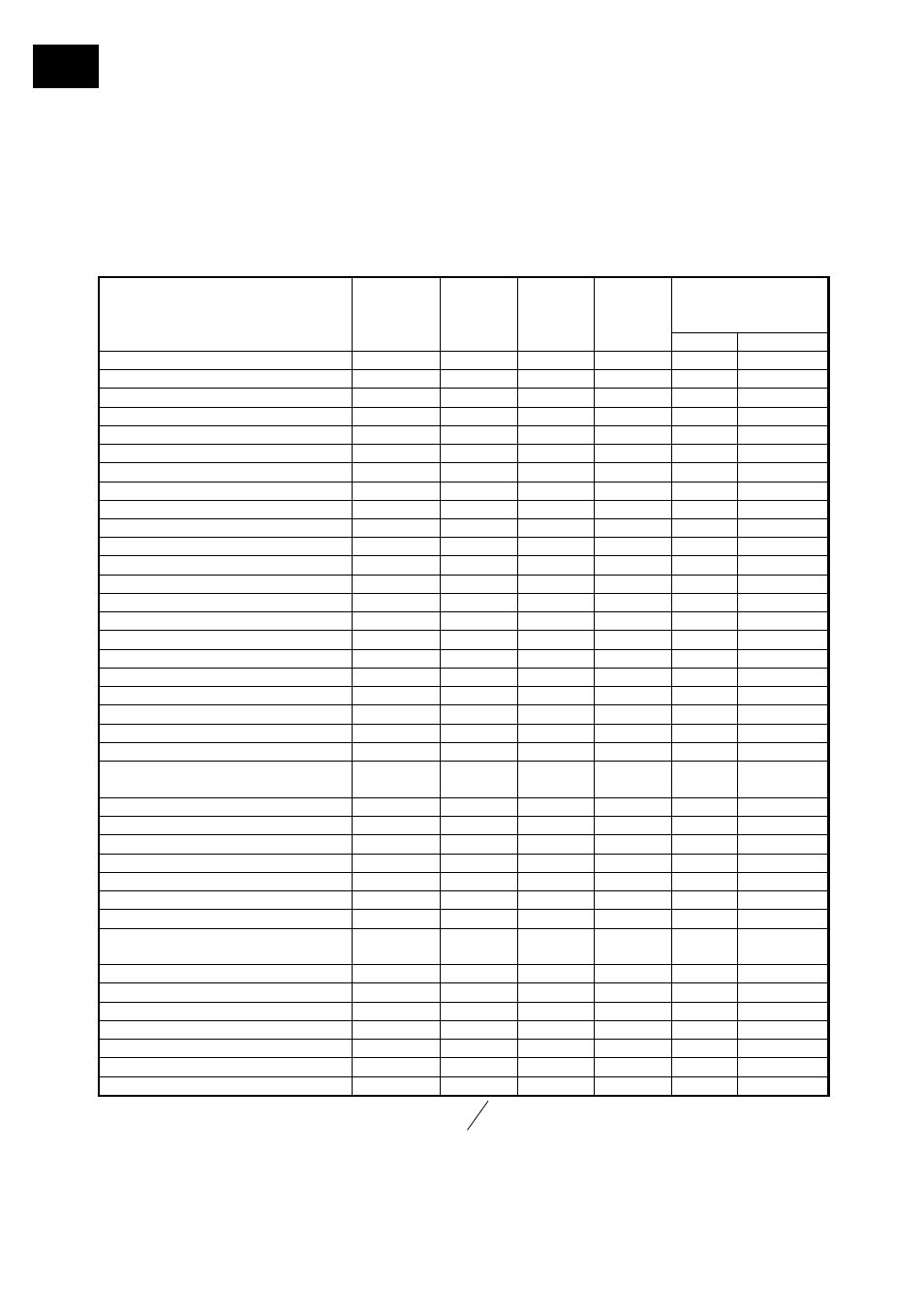
Rise of the straight line is a function of evaporation enthalpy. It is similar for similar
chemical substances with similar boil temperature. Hence, straight lines depicted may be
used for rough reference to a lightly deviating boil point.
A water jet pump or diaphragm-type pump may lower boil temperature by about 100°C.
Rule of thumb: decrease pressure to ½ will decrease boil point by about 15°C.
Vacuum for
Boiling point
at 40°C
Solvent Total
formula
MW
[g/mol]
MW
[°C]
ΔH
vap
[J/g]
[mbar] [mm(Hg)]
Aceton C
3
H
6
O 58,08
56,5 550
556 387
Acetonitril C2H3N
41,05
81,8
833
230
173
Benzol C
6
H
6
78,11
80,1
549
236
177
n-Butanol (Butylalkohol)
C
4
H
10
O 74,12
117,5 619 25 19
tert.-Butanol (tert.-Butylalkohol)
C
4
H
10
O 74,12
82,9 588 130 98
2-Butanon (Methylethylketon)
C
4
H
8
O 72,11
79,6 473
243 182
tert.-Butylmethylether C
5
H
12
O 88,15
55,0
Chlorbenzol C
6
H
5
CI 112,60
132,2 375 36 27
Cyclohexan C
6
H
12
84,16
80,7
389
235
176
1,2-Dichlorethan C
2
H
4
CI
2
98,96 82,4 336 210 158
1,2-Dichlorethylen (cis)
C
2
H
2
CI
2
96,94 59,0 320 479 134
1,2-Dichlorethylen (trans)
C
2
H
2
CI
2
96,94 47,8 313 751 563
Dichlormethan (Methylenchlorid)
CH
2
CI
2
84,93
40,7 373
atm. atm.
Diethylether C
4
H
10
O 74,12
34,6 392 atm. atm.
Diisopropylether C
6
H
14
O 102,20
67,5 318 375 281
Dimethylformamid C
3
H
7
NO 73,09 153,0
11
8
1,4-Dioxan C
4
H
8
O
2
88,11
101,1 406 107 80
Ethanol C
2
H
6
O 46,07
78,4 879
175 131
Ethylacetat C
4
H
8
O
2
88,11
77,1 394 240 180
Heptan C
7
H
16
85,09
98,4
439
120 90
Hexan C
6
H
14
86,18
68,7
370
335
251
Methanol CH
4
O 32,04
64,7
1225
337
253
3-Methyl-1-Butanol
(Isoamylalkohol)
C
5
H
12
O 88,15
130,6 593 14 11
Pentachlorethan C
2
HCI
5
202,30
160,5 203 13 10
Pentan C
5
H
12
72,15
36,1
382
atm.
atm.
n-Pentanol (Amylalkohol)
C
5
H
12
O 88,15
137,8 593 11
8
1-Propanol (n-Propylalkohol)
C
3
H
8
O 60,10
97,8 787 67 50
2-Propanol (Isopropylalkohol)
C
3
H
8
O 60,10
82,5 701
137 103
1,1,2,2-Tetrachlorethan C
2
H
2
CI
4
167,90
145,9 247 35
26
Tetrachlorethylen C
2
CI
4
165,80
120,8
233
53 40
Tetrachlormethan
(Carbontetrachlorid)
CCI
4
153,80
76,7
225
271
203
Tetrahydrofuran C
4
H
8
O 72,11
66,0 357 268
Toluol C
7
H
8
92,14
110,6
425
77 58
1,1,1-Trichlorethan C
2
H
3
CI
3
133,40 74,1 251 300 225
Trichlorethylen C
2
HCI
3
131,40
86,7 265 183 137
Trichlormethan (Chloroform)
CHCI
3
119,40
61,3 263
474 356
Wasser H
2
O 18,02
100,0
2259
72
54
Xylol (Isomeren-Gemisch)
C
8
H
10
106,20
137-143
390
25 19
Conversion factor [mbar] to [mm(Hg)]:
]
[
4
3
)]
(
[
mbar
Hg
mm
≈
