Xi. addendum – Myron L 729II User Manual
Page 71
67
XI. ADDENDUM
A.
CONDUCTIVITY, TDS AND
TEMPERATURE RELATIONSHIPS
1.
TEMPERATURE COMPENSATION
(Tempco) of Aqueous Solutions
Electrical conductivity indicates solution concentration and
ionization of the dissolved material. Since temperature greatly
affects ionization, conductivity measurements are temperature
dependent and are normally corrected to read what they would
be at 25°C.
a. Standardized to 25°C
Conductivity is accurately measured in the 750II by a method
that ignores electrolysis, electrode characteristics, etc., and
uses a unique circuit to perform temperature compensation. In
simpler instruments, conductivity values are usually assigned
an average correction similar to KCl solutions for correction to
25°C. The correction to an equivalent KCl solution is a standard
set by chemists. It standardizes the measurements and allows
calibration with precise KCl solutions recognized for stability.
b. Tempco Variation
Most conductivity instruments use an approximation of the
temperature characteristics of solutions, perhaps even assum-
ing a constant value. The value for KCl is often quoted simply
as 2%/°C. In fact, KCl tempco varies with concentration and
temperature in a non-linear fashion. Other solutions have more
variation still. The 750II uses corrections that change with con-
centration and temperature instead of single average values.
c. An Example of 2 different solution selections
and the resulting compensation:
The 750II will provide the repeatability of data needed for rela-
tive values for process control.
2.
CONDUCTIVITY CONVERSION to TOTAL
DISSOLVED SOLIDS (TDS)
Electrical conductivity indicates solution concentration and
ionization of the dissolved material. Since temperature greatly
affects ionization, conductivity measurements are temperature
dependent and are normally corrected to read what they would
be at 25°C (ref. Temperature Compensation).
a. How it’s Done
Once the effect of temperature is removed, the compensated
conductivity is a function of the concentration (TDS). Tempera-
ture compensation of the conductivity of a solution is performed
automatically by the electronic circuit, using data derived from
chemical tables. Any dissolved salt at a known temperature has
a known ratio of conductivity to concentration. Tables of conver-
sion ratios referenced to 25°C have been published by chemists
for decades.
b. Solution Characteristics
Real world applications have to measure a wide range of materi-
als and mixtures of electrolyte solutions. To solve this problem,
industrial users commonly use the characteristics of a standard
material as a model for their solution, like the KCl favored by
chemists for its stability.
Users dealing with sea water, etc., commonly use NaCl as the
model for their concentration calculations. Users dealing with
freshwater work with mixtures including sulfates, carbonates
and chlorides, the three predominant components (anions) in
freshwater that Myron L Company calls “natural water”. These
are modeled in a mixture called “442™” which the Myron L
Company developed and markets for use as a calibration stan-
dard, as it does standard KCl and NaCl solutions.
c. When does it make a lot of difference?
First, the accuracy of temperature compensation to 25°C deter-
mines the accuracy of any TDS conversion. Assume we have
industrial process water to be pretreated by RO. Assume it is
45°C and reads 1500 µS uncompensated.
1. If NaCl compensation is used, an instrument would report
1035 µS compensated, which corresponds to 510 ppm NaCl.
2. If 442 compensation is used, an instrument would report 1024
µS compensated, which corresponds to 713 ppm 442.
The difference in values is 40%.
In spite of such large error, some users will continue to take
data in the NaCl mode because their previous data gathering
and process monitoring was done with an older NaCl referenced
device.
Those who want true TDS readings that will correspond to
evaporated weight will select the correct Solution Type.
3.
TEMPERATURE COMPENSATION
(Tempco) and TDS DERIVATION
When making conductivity measurements, the Solution Selec-
tion determines the characteristic assumed as the instrument re-
ports what a measured conductivity would be if it were at 25°C.
The characteristic is represented by the tempco, expressed in
%/°C. If a solution of 100 µS at 25°C increases to 122 µS at
35°C, then a 22% increase has happened over this change of
10°C. The solution is said to have a tempco of 2.2 %/°C.
Another solution would have a different tempco because of its
ionization activity. And, that tempco may be a little different at a
different concentration or temperature.
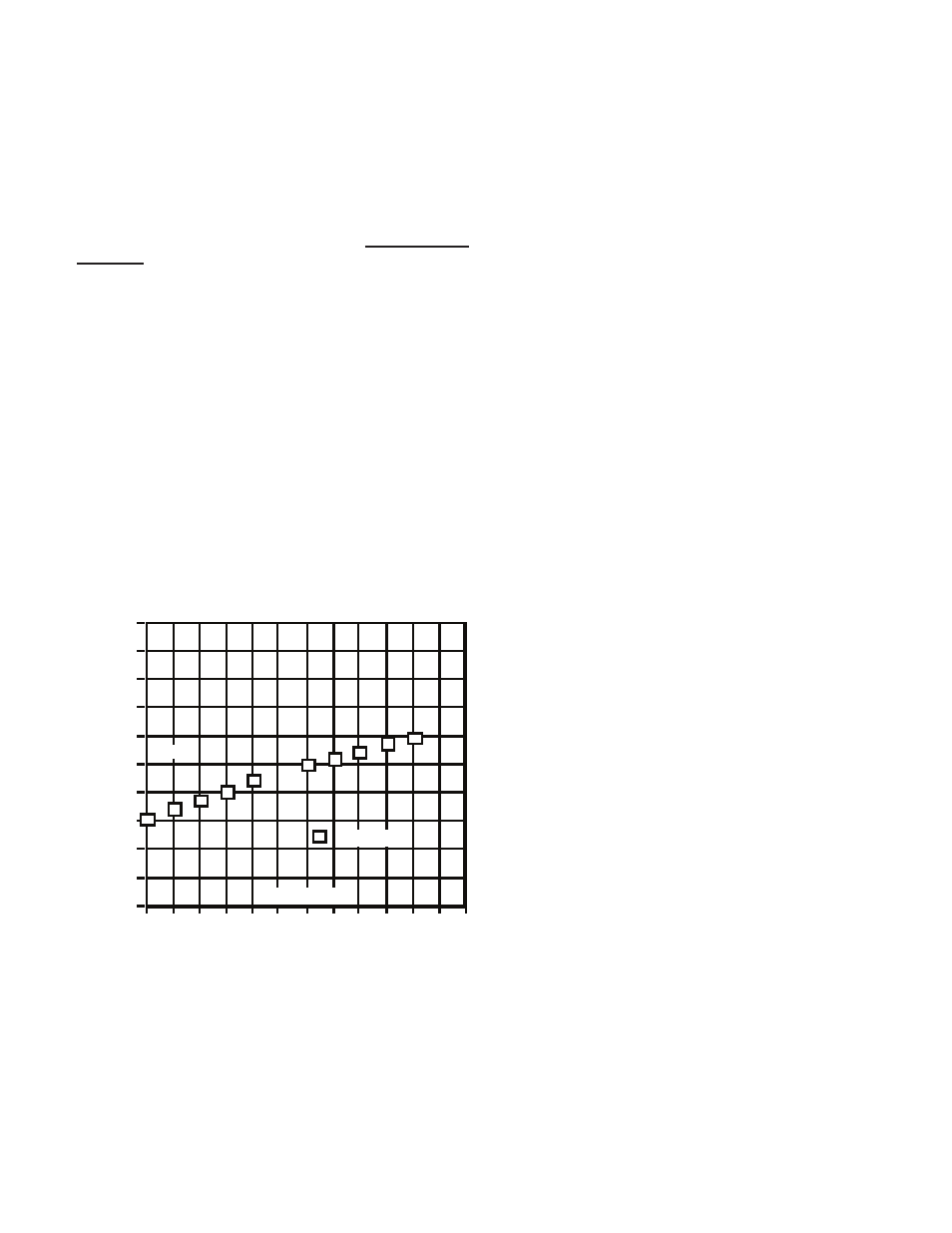
Chart 1
1.500%
1.600%
1.700%
1.800%
1.900%
2.000%
2.100%
2.200%
2.300%
2.400%
2.500%
Temperature
0 5 10 15 20 25 30 35 40 45 50 55 60
KCl % / °C
% / °C
