Merit Medical HepaSphere Microspheres(Bland/Without Drug) IFU-Int'l User Manual
Page 5
5
Note: If the air was previously aspirated from the vial, gentle injection of
air using the syringe prior to aspirating the contents of the vial will ensure
an easier aspiration of vial contents into the syringe. If all contents are
not withdrawn, introduce an additional volume of air and repeat the
aspiration process. It is possible to add an additional amount of non-ionic
contrast or NaCl 0.9% aqueous solution into the syringe in order to get a
higher dispersion of microspheres.
• If microspheres were reconstituted using 100% NaCl 0.9%, non-ionic
contrast medium must be added to the syringe containing the
HepaSphere Microspheres for visualization under fluoroscopy. If non-
ionic contrast medium was used to reconstitute the microspheres,
additional non-ionic contrast medium may be added.
DELIVERY INSTRUCTIONS
• Carefully evaluate the vascular network associated with the target
lesion utilizing high resolution imaging.
Note: It is important to determine if any arteriovenous shunts are
present before beginning embolization.
• Using standard techniques, position the delivery catheter within the
target vessel and the catheter tip as close as possible to the embolization
target.
• Use an injection syringe no larger than 3ml for the delivery of
HepaSphere Microspheres. Use of a 1ml injection syringe is
recommended.
• Aspirate 1ml of the HepaSphere Microspheres mixture into the
injection syringe.
• Two methods for embolic aliquot sequestering for injection may be
used:
- Option 1: Connect a 3 way-stopcock to the 30ml syringe containing
the HepaSphere Microspheres to the infusion micro catheter and use a
1ml syringe for injection through the open port of the 3 way-stopcock.
- Option 2: Serial aliquots of HepaSphere Microspheres can be drawn
from the 30ml syringe into a 1ml injection syringe through a 3 way-stop
cock that is not attached to the infusion catheter. The 1ml syringe
containing each aliquot can be attached independently to the infusion
microcatheter and injected.
• Invert the 30ml syringe back and forth to maintain the homogenous
suspension of the HepaSphere Microspheres mixture.
• Under continuous fluoroscopic guidance, inject the aliquot of
HepaSphere Microspheres in a slow, non forceful, pulsatile manner over
a time period of approximately 1 minute per ml of microspheres solution.
Always inject under free-flow conditions and monitor for reflux.
Note: Reflux of embolic spheres can induce immediate ischemia of
untargeted tissues and vessels.
• When stasis in the feeding pedicle occurs while delivering the
HepaSphere Microspheres, wait a minimum of 5 minutes then perform a
selective angiogram after the full 5 minutes wait to verify the cessation
of antegrade flow.
• If cessation of antegrade flow has not occurred, continue infusion
under fluoroscopic guidance until the desired devascularization is
obtained.
• After the HepaSphere Microsphere infusion is completed, remove the
catheter while maintaining gentle aspiration to avoid dislodging any
residual HepaSphere Microspheres that may still be in the catheter
lumen. Discard the catheter after removal and do not reuse.
• Discard any open vial or unused HepaSphere Microspheres.
CAUTION
In the event that the catheter becomes obstructed or significant infusion
resistance is encountered during injection, do not attempt to flush the
catheter with excessive pressure because reflux of embolic material may
occur resulting in untargeted embolization. Remove the catheter while
applying gentle aspiration and discard.
CONSERVATION AND STORAGE
HepaSphere Microspheres must be stored in a dry, dark place in their
original vials and packaging. Use by the date indicated on the labels of
the outer box and pouch.
When the procedure of reconstitution is completed, store the solution of
HepaSphere Microspheres in 2 to 8°C conditions and use within 24
hours, IF not used immediately. Do not store HepaSphere Microspheres
after contrast medium has been added.
Information on packaging:
All serious or life threatening adverse events or deaths associated
with use of HepaSphere Microspheres should be reported to the
device manufacturer.
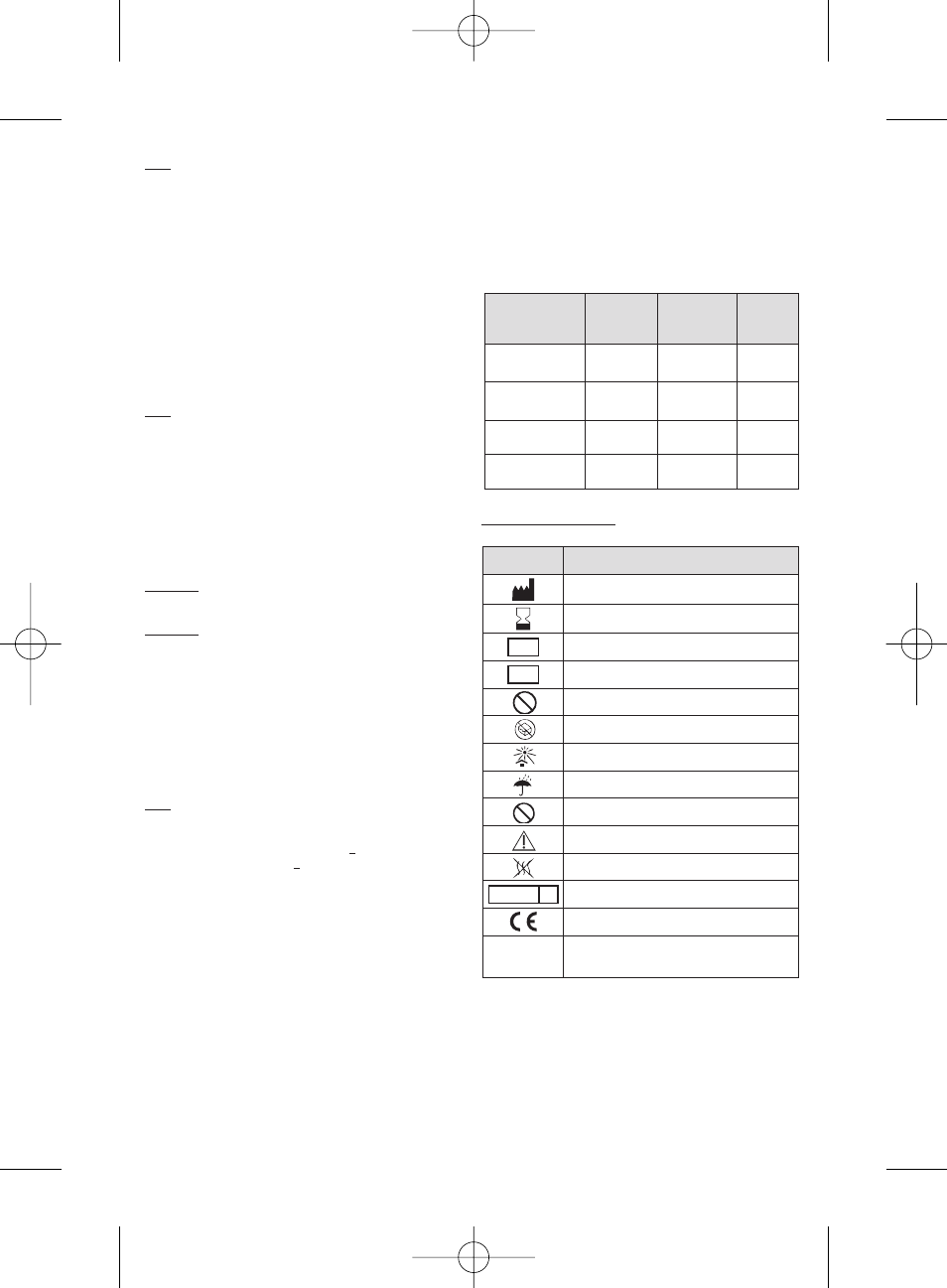
Size of dry products
(µm)
Colour code
(label
borders)
Quantity of
microspheres
(mg)
Reference
30-60
Orange
25
50
V 225 HS
V 250 HS
50-100
Yellow
25
50
V325 HS
V350 HS
100-150
Blue
25
50
V525 HS
V550 HS
150-200
Red
25
50
V725 HS
V750 HS
Symbol
Designation
Manufacturer: Name & Address
Use by date: year-month
LOT
Batch code
REF
Catalogue number
STERILIZE
2
Do not resterilize
Do not use if package is damaged
Keep away from sunlight
Keep dry
2
Do not re-use
Caution - Refer to Instructions For Use
Non-pyrogenic
STERILE R
Sterilized using irradiation
EC mark logo - Notified body identification : 0459
Size of dry microspheres / Size of hydrated
microspheres
•/
•
730070003_A ID 102412 _ HS Bland IFU:Mise en page 1 12/11/12 9:58 Page 5
