Bio-Rad Nuvia™ IMAC Resin User Manual
Page 36
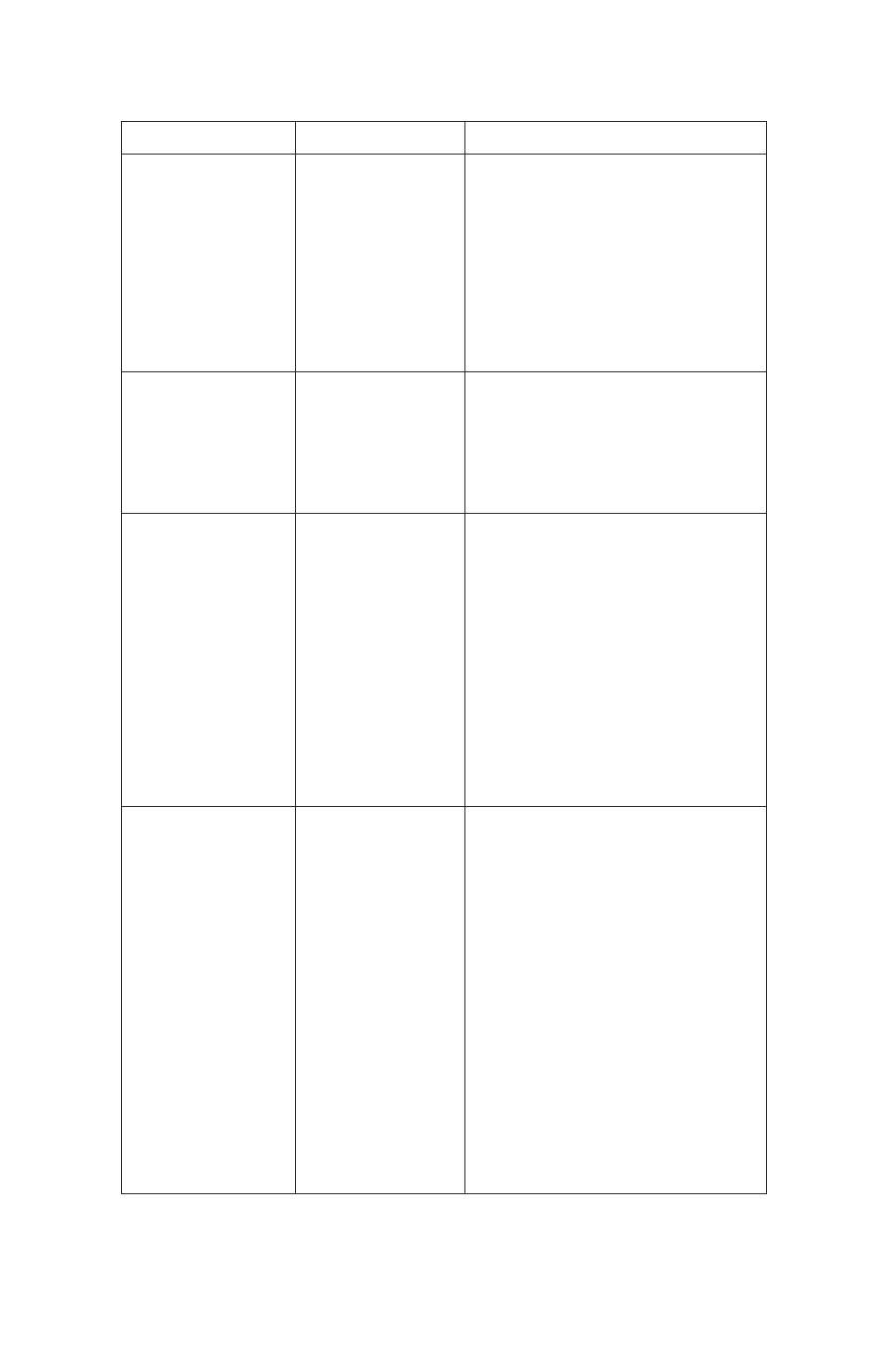
32 Nuvia IMAC Ni-Charged Resin
Problem
Possible Cause
Solution
No protein is eluted
(continued from
previous page)
Target protein is found
in the flowthrough
(continued from
previous page)
Elution conditions
are too mild or
protein may be in
an aggregated or
multimer form
Proteolytic cleavage during fermentation
or purification has caused the histidine tag
to be removed. Add protease inhibitors or
make a new construct with histidine tag
attached to other terminus
Elute with pH or imidazole step elution
Protein precipitates
during purification
Temperature is too
low
Aggregate forms
Perform the purification at room
temperature
Add solubilization agents to samples and/
or buffers: 0.1% Triton X-100, Tween 20,
20 mM
β-mercaptoethanol and ≤20%
glycerol to maintain protein solubility
Poor recovery
of target protein
Protein is found in the
flowthrough
Binding capacity of
the column has been
exceeded
Target protein was
not detected in the
flowthrough
Strong nonspecific
adsorption of the
target protein to the
matrix
See recommendations in No protein is
eluted section
Increase the column size or reduce the
sample volume application
Capillary sample loop is too small
Reduce hydrophobic adsorption by
including detergents or organic solvents,
or by increasing the concentration of NaCl
Histidine-tagged
protein is not pure
Contaminants elute
with target protein
Strongly bound
contaminants elute
with protein
Association of
contaminating
proteins with target
protein via disulfide
bonds
Make binding and wash steps more
stringent. Include 10–20 mM imidazole in
binding and wash buffers
Prolong the wash step containing
imidazole
Column is too large; reduce amount of
Nuvia
™
IMAC resin used
Very high concentrations of imidazole
will cause strongly bound contaminants
to elute as well. Reduce the imidazole
concentration during the elution
Include ≤30 mM
β-mercaptoethanol.
Exercise caution if using DTT
