Appendix, Appendix 7.1 orp as a function of mv – KROHNE OPTISENS ORP 8590 EN User Manual
Page 30
7
APPENDIX
30
OPTISENS ORP 8590
www.krohne.com
01/2014 - 4001933801 MA OPTISENS ORP 8590 R01 en
Appendix
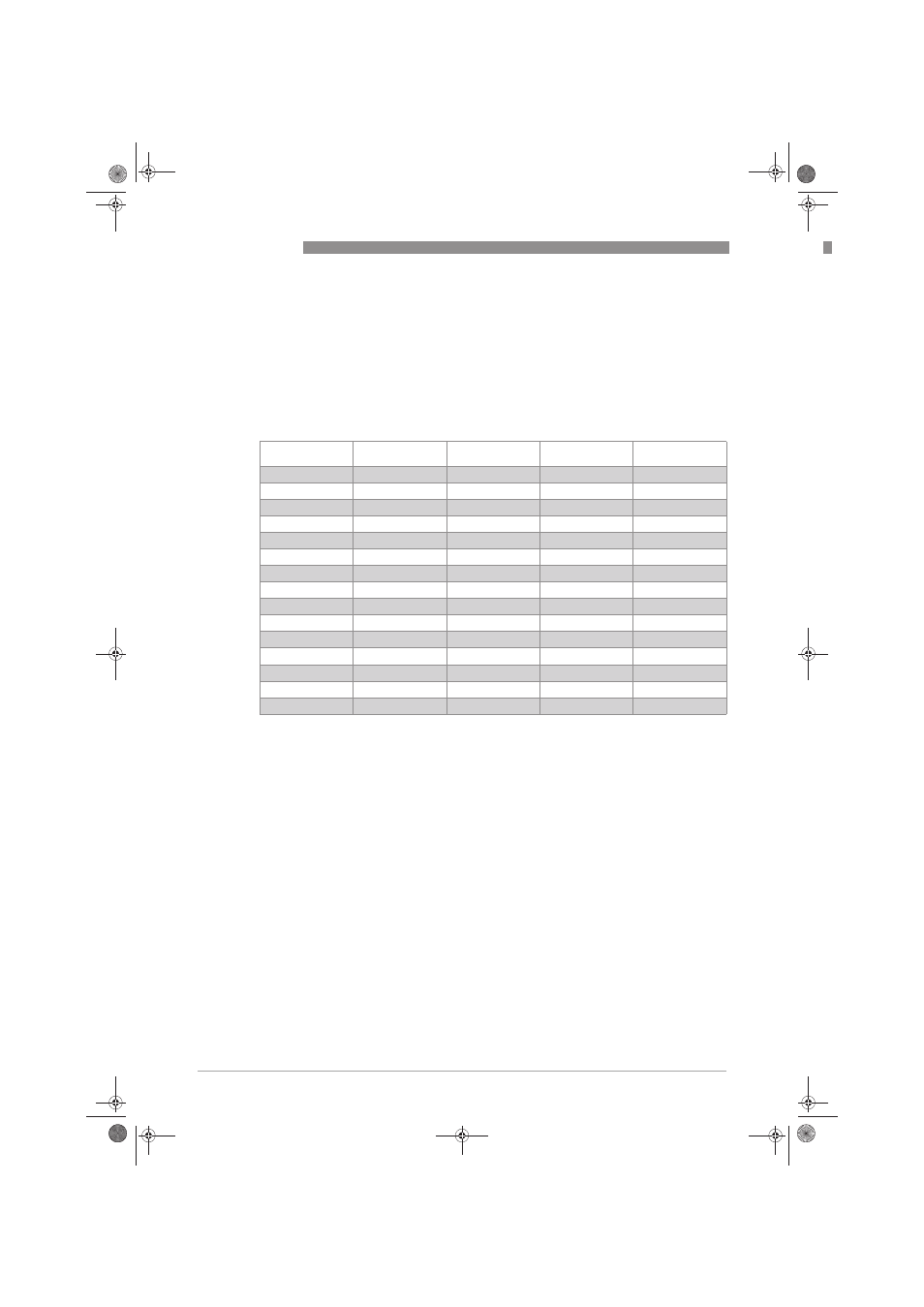
7.1 ORP as a function of mV
The pH-value is the negative decadative logarithm of the hydrogen ion concentration, and it is
directly related to the proportion of hydrogen ions H
+
to hydroxide ions OH
-
in the media. The pH-
sensor measures excess or deficit of the hydrogen ions and gives a proportional millivolt signal
as output. The signal is 59.16 mV per 1 pH at 25°C / 77°F. In clean water there is a total balance
between hydrogen ions and hydroxide ions, the output from the electrode is 0.0 mV and pH is 7.
The millivolt signal is measured by the pH sensor and the corresponding pH value is calculated
in the signal converter.
mV
pH
H
+
ions [mol/l]
OH
-
ions [mol/l]
Example
414
0
1
0.00000000000001
355
1
0.1
0.0000000000001
296
2
0.01
0.000000000001 Coca Cola
237
3
0.001
0.00000000001
177
4
0.0001
0.0000000001 Orange juice
118
5
0.00001
0.000000001
59
6
0.000001
0.00000001 Milk
0
7
0.0000001
0.0000001 Clean water
-59
8
0.00000001
0.000001 Blood
-118
9
0.000000001
0.00001
-177
10
0.0000000001
0.0001
-237
11
0.00000000001
0.001
-296
12
0.000000000001
0.01
-355
13
0.0000000000001
0.1
-414
14
0.00000000000001
1 Sulfa
.book Page 30 Friday, January 24, 2014 11:29 AM
