Gas theory – GxT V016-01, Ferret 16 GasLink II 5-Gas Analyzer User Manual
Page 17
17
Gas Theory
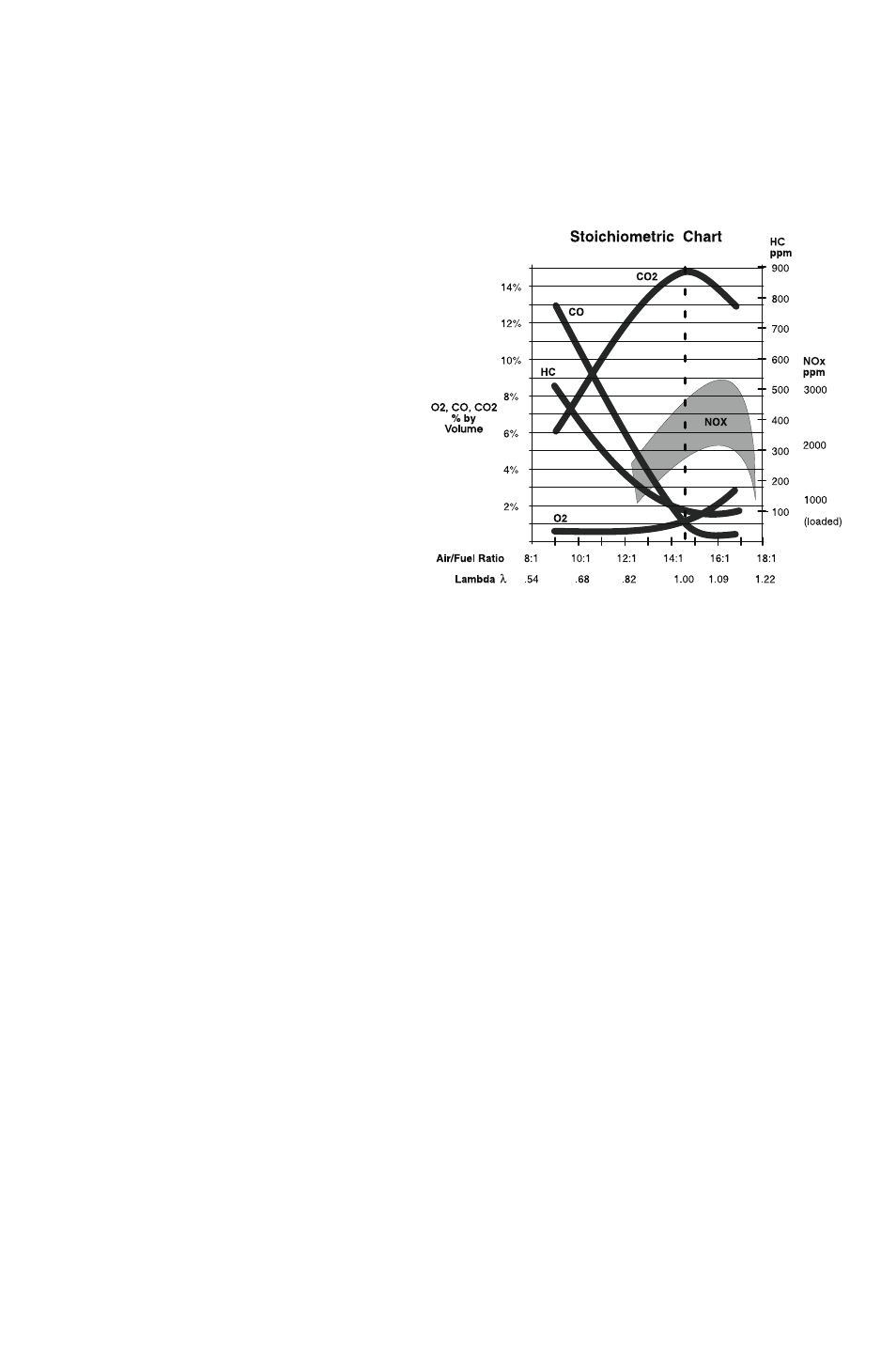
The content of an engine’s exhaust stream depends on the efficiency of the
combustion process. Ideally, pure fuel (hydrogen and carbon) and oxygen
would enter the combustion chamber in precisely the right amounts (called
stoichiometry), and the mixture
would be ignited at just the
right moment so that all of the
oxygen would combine with
all of the fuel. In this perfect
example, the hydrogen (H) in
the fuel would combine with
oxygen (O2) to form water
(H2O) and the carbon (C) in
the fuel would combine with
oxygen to form carbon dioxide
(CO2). Those two compounds
and heat would be the only
products of combustion. Com-
bustion would be complete.
In reality, the air we breath and the fuel used are not pure, and the spark
may not occur at precisely the right moment. Subsequently, the exhaust
stream will contain hydrocarbons (HC), and carbon monoxide (CO), in
addition to Carbon Dioxide (CO2) and traces of Oxygen (O2), Oxides of
Nitrogen (NOx), Sulfur Dioxides (SO2) and soot.
The analyzer will measure HC, CO, CO2, O2, and NOx levels in the
exhaust stream. The relative amounts of these compounds will provide
information about the combustion process and clues about the causes of
abnormal levels.
There are a lot of things that can go wrong with an engine, and understand-
ing the results of different component failures will go a long way toward
helping you use your analyzer to its full potential.
