Equilibrium diagram, General information – Carrier 16JA User Manual
Page 10
Attention! The text in this document has been recognized automatically. To view the original document, you can use the "Original mode".
GENERAL INFORMATION
EQUILIBRIUM DIAGRAM
The Equilibrium Diagram (Fig. 5) is used to
determine solution concentration in your machine.
It is important to maintain solution concentration
within certain limits in order to maintain equilib
rium conditions.
The following explains the equilibrium diagram
and how to determine solution concentration:
The curved line in the lower right-hand corner
is the crystallization line. This line indicates the
point at which the solution will begin to change
from a liquid to a solid. This sets the limits of
the cycle. Crystallization of a solution is quite
different from the freezing of a single substance
such as water. When water is subjected to a
temperature even slightly below 32 F, all of it
will eventually freeze. In contrast, when the
lithium bromide solution temperature is reduced
below the solidification point for that particular
concentration, only a portion of the salt will
crystallize or freeze. The remainder of the solu
tion will become more dilute or less concentrated
and will remain in a liquid state. Crossing of the
crystallization line does not necessarily result in
solidification provided the subcooling does not
progress too far. Solidification of solution will
not harm the absorption machine but it will
interrupt service. Satisfactory design requires
that operation take place above the crystalliza
tion line.
The scale on the left represents the straight
horizontal lines and indicates the vapor pressure
of the solution or evaporator water at equilib
rium conditions.
On the right-hand side is the saturation temper
ature scale for pure water corresponding to the
vapor pressures on the left-hand scale. This
scale also represents the horizontal lines and
is located on the right side to avoid confusion in
reading the chart.
The scale at the bottom is for the vertical
lines. They represent solution concentration in
percent by weight. For example, a solution of
60% is 60% lithium bromide and 40% water
by weight.
The curved lines running from left to right
are solution temperature lines. These should not
be confused with the saturation temperatures.
The curved lines which extend upward from the
bottom of the diagram are specific gravity lines.
These are used to determine solution concentra
tion. By measuring the specific gravity with a
hydrometer and finding the temperature, the per
cent of concentration can be determined by plot
ting these two points on the diagram.
Refer to the typical machine absorption cycle
plotted on Fig. 5, Points 1 thru 7 represent a
complete cycle. Specific point values are given
in Table 1. An explanation of each point and the
lines drawn between is as follows:
Point 1 - The strong solution as it sprays out of
the absorber spray nozzle and starts to
absorb refrigerant.
Point 2 - The weak solution as it leaves the ab
sorber and enters the heat exchanger.
Line 1-2 represents absorption of the
refrigerant thereby diluting solution.
Point 3 - The weak solution after it has passed
thru the heat exchanger. Line 1-3 rep
resents the amount of heat gained by
the solution in the heat exchanger.
Point 4 - The weak solution entering the genera
tor and being heated. Line 3-4 repre
sents the amount of heat required to
start the weak solution to boil.
Point 5 - Maximum solution concentration in the
generator after much of the refrigerant
has boiled out. Line 4-5 represents the
amount of heat required to boil off the
refrigerant.
Point 6 - The strong solution as it leaves the heat
exchanger on its way to spray nozzles.
Point 7 - The strong solution entering the spray
nozzles.
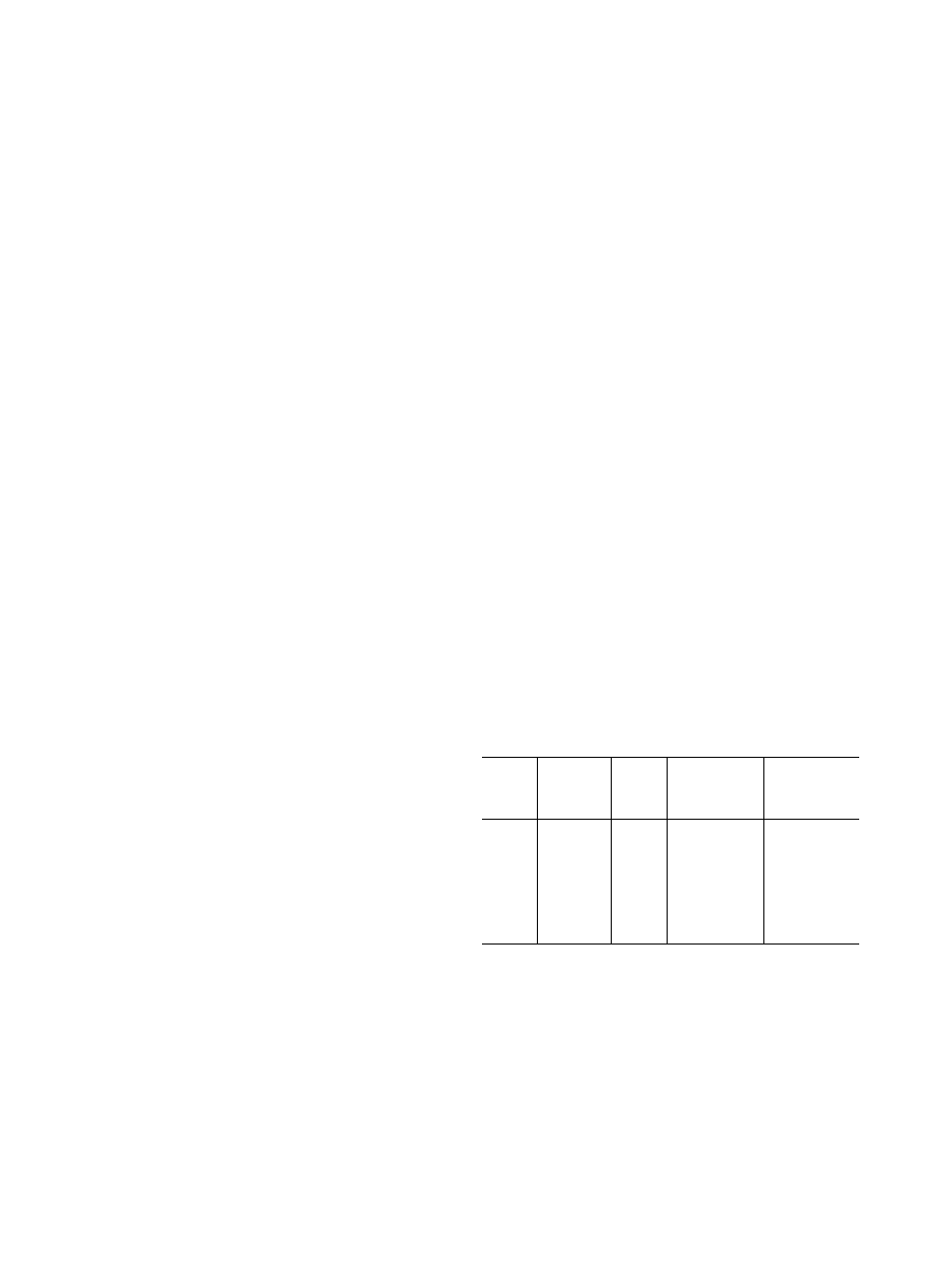
Table 1 - Cycle Data
POINT
SOLUTION
TEMP
(F)
VAPOR
PRESS.
(in Hg)
PERCENT
LITHIUM
BROMIDE SOL
SATURATED
TEMP
(F)
1
115
0 25
63.3
42
2
101
0 25
59 5
42
3
165
1 65
59 5
95
4
192
3.20
59.5
115
5
215
3 20
64 0
115
6
134
0.45
64 0
55
7
119
0.30
63 0
45
10
