Calculation of alkalinity relationships – LaMotte AM-21 Water Pollution Detection Outfit User Manual
Page 8
Calculation of Alkalinity Relationships
The results obtained from the phenolphthalein and total alkalinity
determination offer a means for the stoichiometric classification of the three
principal forms of alkalinity present in many water supplies. The
classification ascribes the entire alkalinity to bicarbonate, carbonate, and
hydroxide; and assumes the absence of other weak acids of inorganic or
organic composition, such as silicic, phosphoric, and boric. This
classification system further presupposes the incompatibility of hydroxide
and bicarbonate alkalinities in the same sample. Since the calculations are
on a stoichiometric basis, ion concentrations in the strictest sense are not
represented in the results.
Carbonate alkalinity is present when the phenolphthalein alkalinity is not
zero but is less than the total alkalinity.
Hydroxide alkalinity is present if the phenolphthalein alkalinity is more than
one-half the total alkalinity.
Bicarbonate alkalinity is present if the phenolphthalein alkalinity is less than
one-half the total alkalinity.
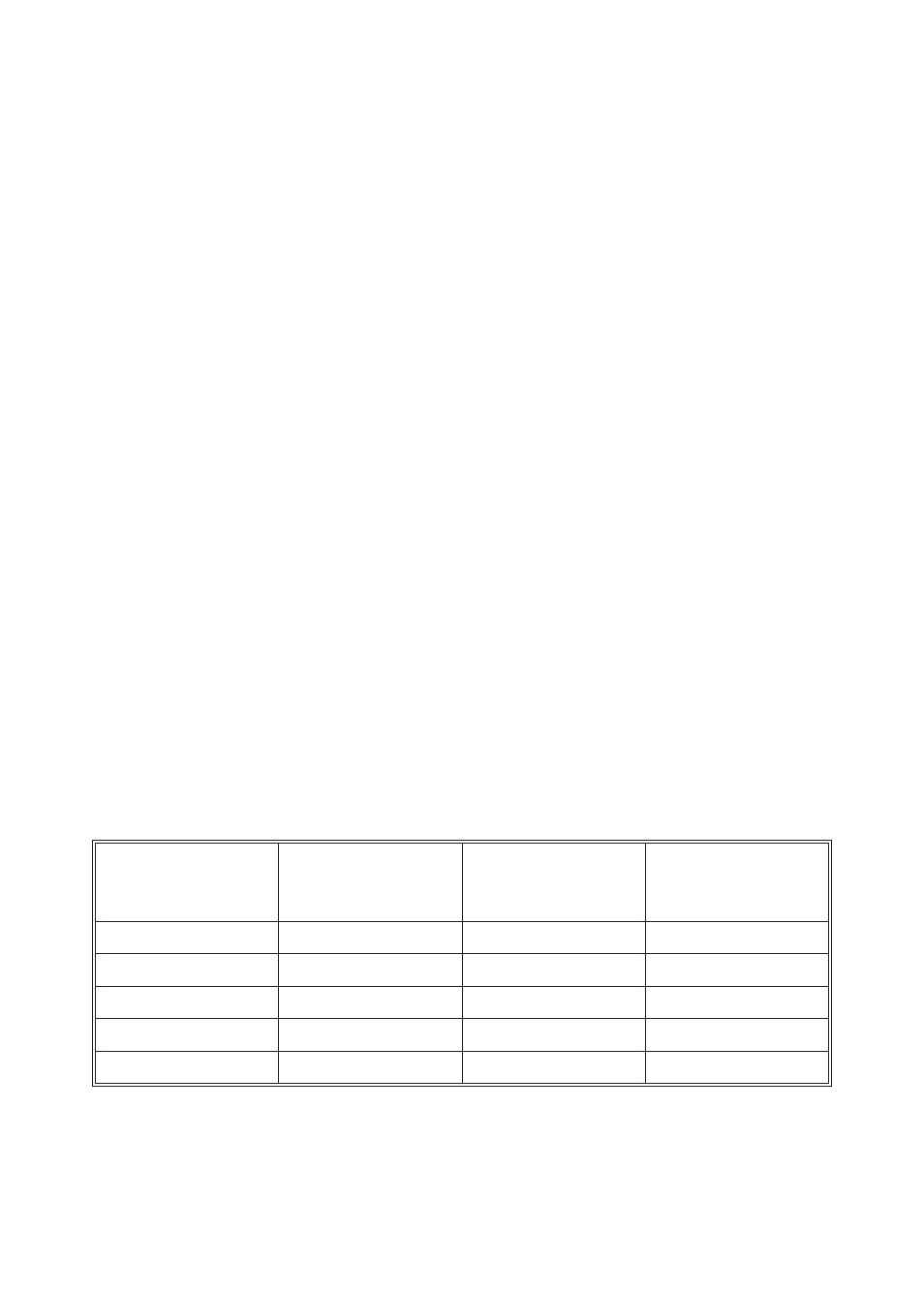
The mathematical conversion of the results is shown in the following table:
Relationships Between Phenolphthalein Alkalinity, Total Alkalinity,
Carbonate Alkalinity, And Hydroxide Alkalinity:
Result of
Titration
Hydroxide
Alkalinity
as CaCO
3
Carbonate
Alkalinity as
CaCO
3
Bicarbonate
Alkalinity
as CaCO
3
P=0
0
0
T
P<½T
0
2P
T-2P
P=½T
0
2P
0
P>½T
2P-T
2(T-P)
0
P=T
T
0
0T
8
