Alkalinity test – LaMotte AM-21 Water Pollution Detection Outfit User Manual
Page 6
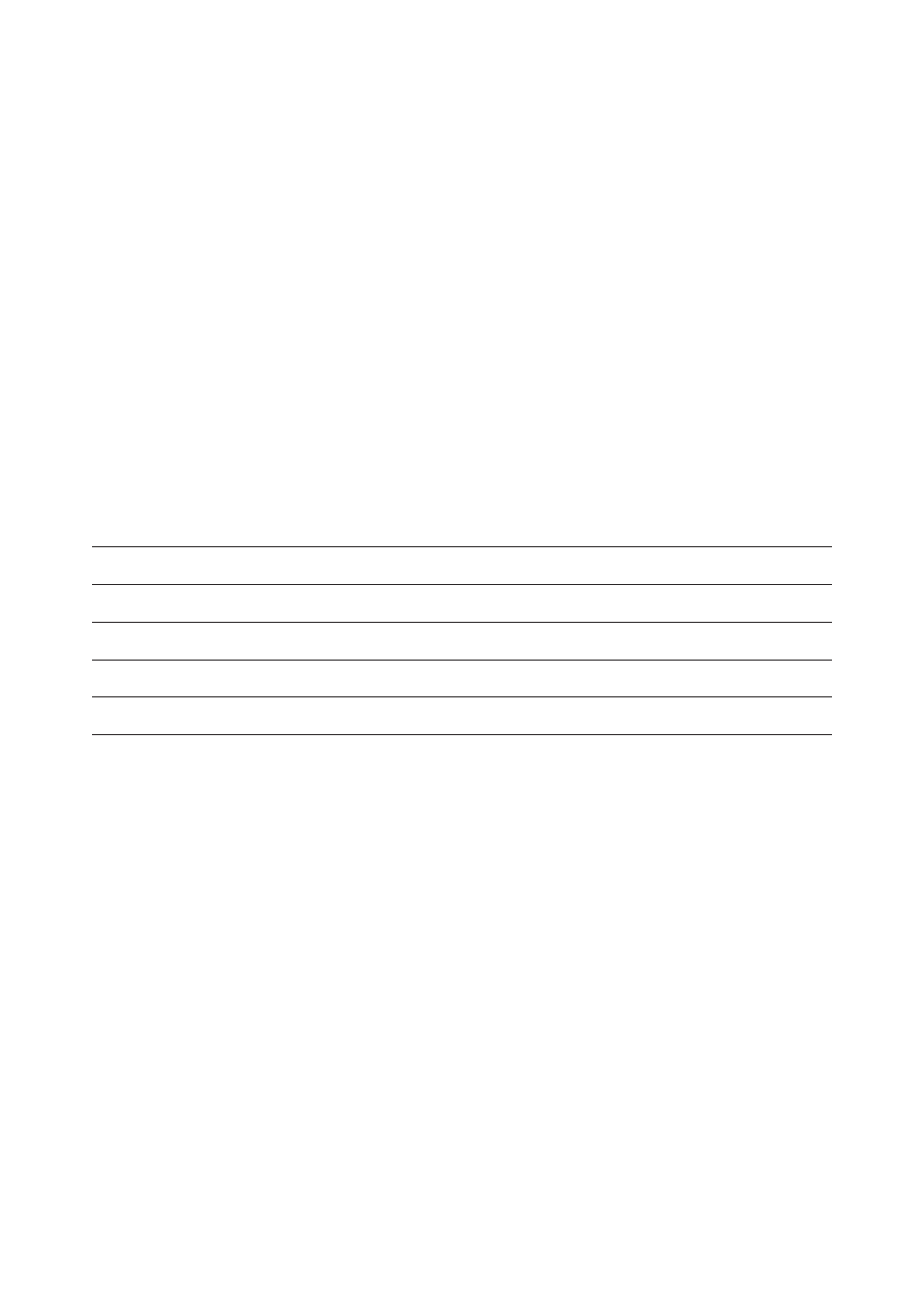
Alkalinity Test
The normal conditions of the alkalinity of natural waters are associated with
the carbon dioxide, bicarbonate, carbonate and hydroxide components.
These factors are characteristic of the source of water and the natural
processes taking place at any given time. For particular industrial and
domestic use, it is often desirable to change these characteristics by
treatments such as aeration, neutralization, softening, etc. The particular
treatment and the extent to which it is employed will depend upon the end
use of the water.
Alkalinity of a water is determined by titration with a standard acid to
successive indicator endpoints, thus permitting the calculation of the various
forms of alkalinity.
Field Test Method
Quantity
Contents
Code
15 mL
Total Alkalinity Indicator
2786-E
100
Phenolphthalein Tablets
T-2246-J
30 mL
*Alkalinity Titration Reagent B
*4493-G
1
Test Tube, 5-10-15 mL, glass, w/cap
0778
1
Direct Reading Titrator, 0-200 Range
0382
WARNING: Reagents marked with a * are considered to be potential health hazards.
To view or print a Material Safety Data Sheet (MSDS) for these reagents see MSDS CD
or www.lamotte.com. To obtain a printed copy, contact LaMotte by email, phone or fax.
The alkalinity titration tube is calibrated so that the result can be read
directly from the scale on the titrator in ppm calcium carbonate (CaCO
3
).
The result can be translated to grains per gallon by multiplying the reading
by the factor 0.0585.
Read the LaMotte Direct Reading Titrator Manual before prceeding.
6
