Experiment 1: the electrical equivalent of heat – PASCO TD-8552 ELECTRICAL EQUIVALENT OF HEAT User Manual
Page 7
012-02833D
Electrical Equivalent of Heat
3
Experiment 1: The Electrical Equivalent of Heat
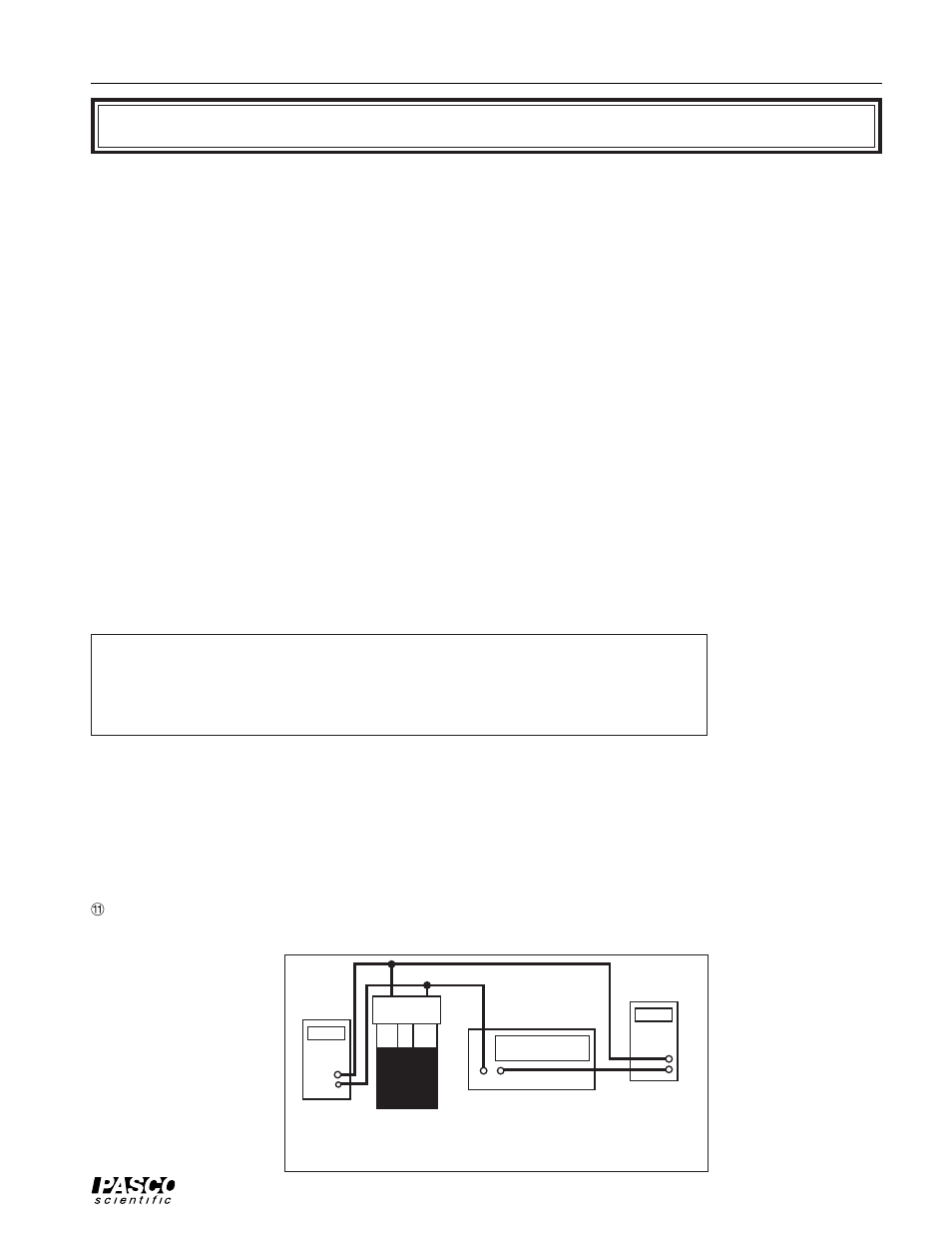
Figure 1.1 Electrical Connections
+ –
+
–
+
–
Power Supply
Voltmeter
Ammeter
13 V Max!
➀
Measure and record the room temperature (T
r
).
➁ Weigh the EEH Jar (with the lid on), and record its mass (M
j
).
➂ Remove the lid of the EEH Jar and fill the jar to the indicated water line with cold wa-
ter. DO NOT OVERFILL. The water should be approxmately 10°C below room tem-
perature, but the exact temperature is not critical.
➃ Add about 10 drops of India ink to the water; enough so the lamp filament is just
barely visible when the lamp is illuminated.
➄ Using leads with banana plug connectors, attach your power supply to the terminals of
the EEH Jar. Connect a voltmeter and ammeter as shown in Figure 1.1 so you can mea-
sure both the current (I) and voltage (V) going into the lamp. NOTE: For best results,
connect the voltmeter leads directly to the binding posts of the jar.
➅ Turn on the power supply and quickly adjust the power supply voltage to about 11.5
volts, then shut the power off. DO NOT LET THE VOLTAGE EXCEED 13
VOLTS.
➆ Insert the EEH Jar into one of the styrofoam Calorimeters.
➇ Insert your thermometer or thermistor probe through the hole in the top of the EEH Jar.
Stir the water gently with the thermometer or probe while observing the temperature.
When the temperature warms to about 6 or 8 degrees below room temperature, turn the
power supply on.
➤ NOTE: You may want to turn the lamp on to help the cold water reach this
starting temperature. If you do, be sure that you turn the lamp off for several minutes
before you begin your measurements, so you are sure the water temperature is even
throughout the jar. Record the starting time (t
i
) and the temperature (T
i
).
➈ Record the current, I, and voltage, V. Keep an eye on the ammeter and voltmeter
throughout the experiment to be sure these values do not shift significantly. If they do
shift, use an average value for V and I in your calculations.
➉ When the temperature is as far above room temperature as it was below room tempera-
ture (T
r
- T
i
= Temperature - T
r
), shut off the power and record the time (t
f
). Continue
stirring the water gently. Watch the thermometer or probe until the temperature peaks
and starts to drop. Record this peak temperature (T
f
).
Weigh the EEH Jar with the water, and record the value (M
jw
).
