Introduction, Equipment – PASCO TD-8552 ELECTRICAL EQUIVALENT OF HEAT User Manual
Page 5
012-02833D
Electrical Equivalent of Heat
1
Introduction
The PASCO Model TD-8552 Electrical Equivalent of
Heat Apparatus provides an experimental determina-
tion of the quantitative relationship between electrical
energy and heat. Conversely, if the electrical equiva-
lent of heat is accepted as a given, this apparatus can
provide a convincing demonstration of the conserva-
tion of energy. With either approach, the experiment
is easily extended to determine the energy efficiency
of an incandescent lamp.
Instructions for two experiments, along with student
worksheets, are on pages 3-6. In Experiment 1, the Electri-
cal Equivalent of Heat is experimentally determined. An
incandescent lamp is immersed in a known quantity of water
and a few drops of India ink are added to the water so it is
opaque to visible light. The temperature of the water is
measured. The lamp is then illuminated with a fixed current
and voltage for a measured time interval, so the electrical
energy into the lamp can be calculated. By monitoring the
temperature of the water, the heat produced by the lamp can
also be calculated. The ratio between the electrical energy
that flows into the lamp and the heat produced by the lamp
determines the electrical equivalent of heat.
In Experiment 2, the efficiency of the incandescent lamp is
measured. The details are similar to Experiment 1, but no
india ink is added to the water. Without the ink, the thermal
energy and infrared radiation from the lamp are absorbed
into the water, but the visible light escapes. To determine
the amount of energy that was released as light, the heat
transferred into the water is subtracted from the total
electrical energy that flowed into the lamp . The ratio
between the light energy and the electrical energy gives the
efficiency of the bulb.
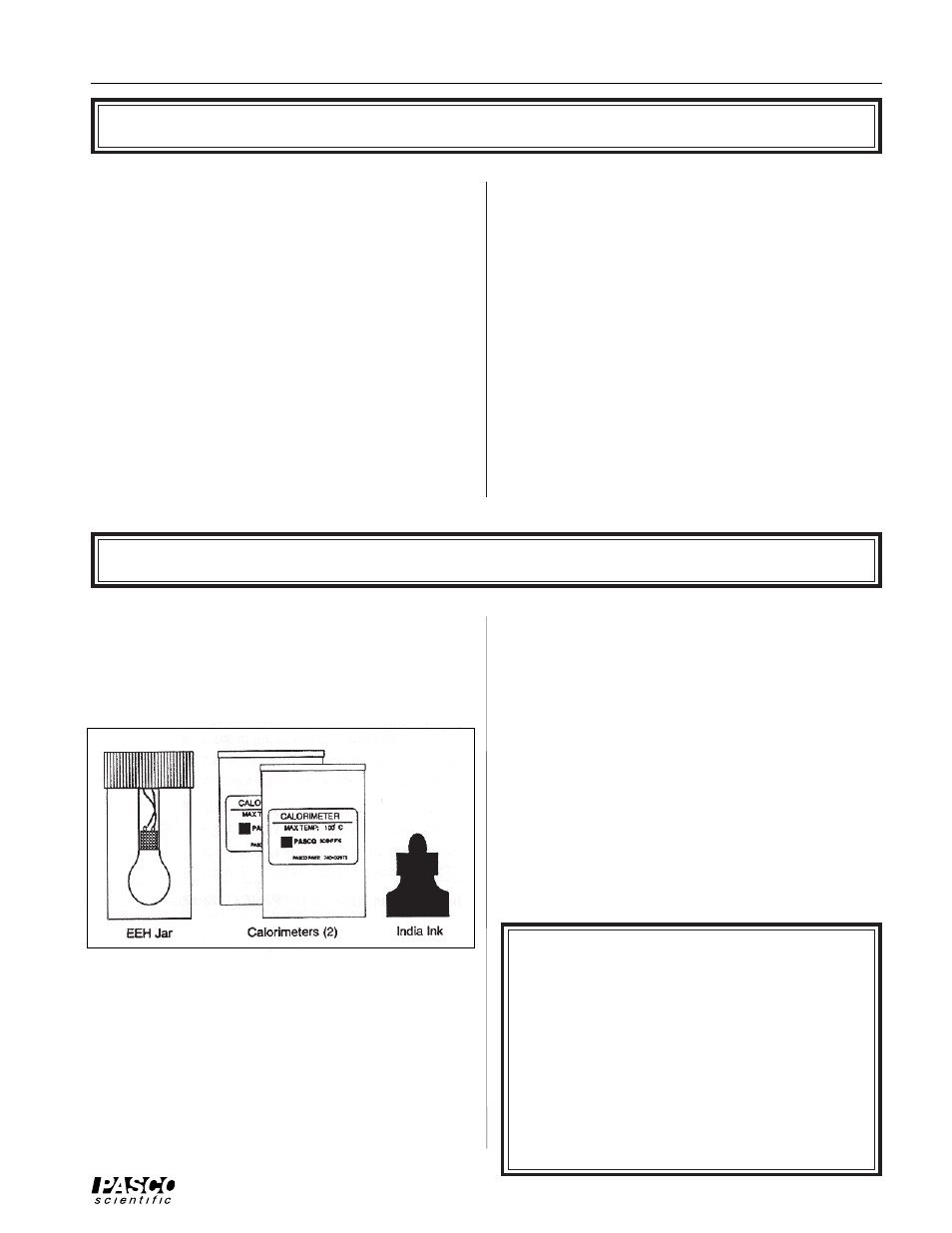
Equipment
Your Model 8552 Electrical Equivalent of Heat
apparatus includes the items shown in Figure 1: a
transparent Electrical Equivalent of Heat Jar (EEH Jar)
with a built-in 35 Watt incandescent lamp, two
styrofoam Calorimeters, and a bottle of India ink.
➁ A digital Volt-Ammeter (a separate voltmeter and
ammeter are best) for measuring the power input to
the lamp. (Such as PASCO Model SE-9589.)
➂ A clock or stopwatch to determine the electrical en-
ergy that flows into the lamp (energy = power x
time).
➃ A thermometer, or PASCO's TD-8559 Thermistor
Probe.*
➄ A balance for accurately determining the mass of
the water heated by the bulb.
(* A digital ohmmeter (SE-9589) is recom-
mended for use with the Thermistor Probe.)
➤ IMPORTANT: When using the Electrical
Equivalent of Heat Apparatus, always observe
the following precautions:
➀ Do not fill the water beyond the line indicated
on the EEH Jar. Filling beyond this level can
significantly reduce the life of the lamp.
➁ Illuminate the lamp only when it is immersed
in water.
➂ Never power the incandescent lamp at a
voltage in excess of 13 V.
Additional Equipment Needed:
In addition to the equipment included with your
Electrical Equivalent of Heat apparatus, you will need
the following items to perform the experiments in this
manual:
➀ A regulated power supply capable of delivering up
to 3 A at 12 V. (Such as PASCO Model SF-9584.)
Figure 1 Equipment
