Operation seebeck effect, The change in entropy of the hot water, The change in entropy of the cold water – PASCO TD-8550A THERMOELECTRIC CONVERTER User Manual
Page 2: Once the water is mixed, however, t, S = q/t , where
012-04929A
2
scientific
Figure 2
➀
The change in entropy of the hot water,
∆
S
h
= Q
h
/
T
h
, is negative, because of the heat transfer from
the water into the Converter.
➁
The change in entropy of the cold water,
∆
S
c
= Q
c
/T
c
, is positive, because of the heat trans-
fer from the Converter into the water.
➂
According to the second law, the total change in
entropy,
∆
S
T
=
∆
S
c
+
∆
S
h
, must be positive. There-
fore, the process will only take place if Q
c
/T
c
> Q
h
/
T
h
.
In order for the fan to be turned, some of the heat
transferred from the hot water must be converted
into work and will therefore not be available to be
transferred back into the cold water. Therefore,
whenever the fan turns, Q
h
> Q
c
.
➄
The equations in steps 3 and 4 can only both be true
if T
h
> T
c
. Once the water is mixed, however, T
h
=
T
c
. Therefore, if the fan were to turn, it would vio-
late the Second Law of Thermodynamics.
This was Kelvin’s statement of the Second Law of
Thermodynamics. The second law has been stated in
many, seemingly unrelated ways; but in the end, all
have been shown to be different ways of expressing
the same basic principle. In its most general form, the
Second Law tells us that no physical process will
occur if it decreases the disorder—or entropy—of the
universe. Conservation of energy, as expressed in the
First Law of Thermodynamics, holds for every physi-
cal process. But many processes which would con-
serve energy do not occur. The Second Law describes
this phenomenon.
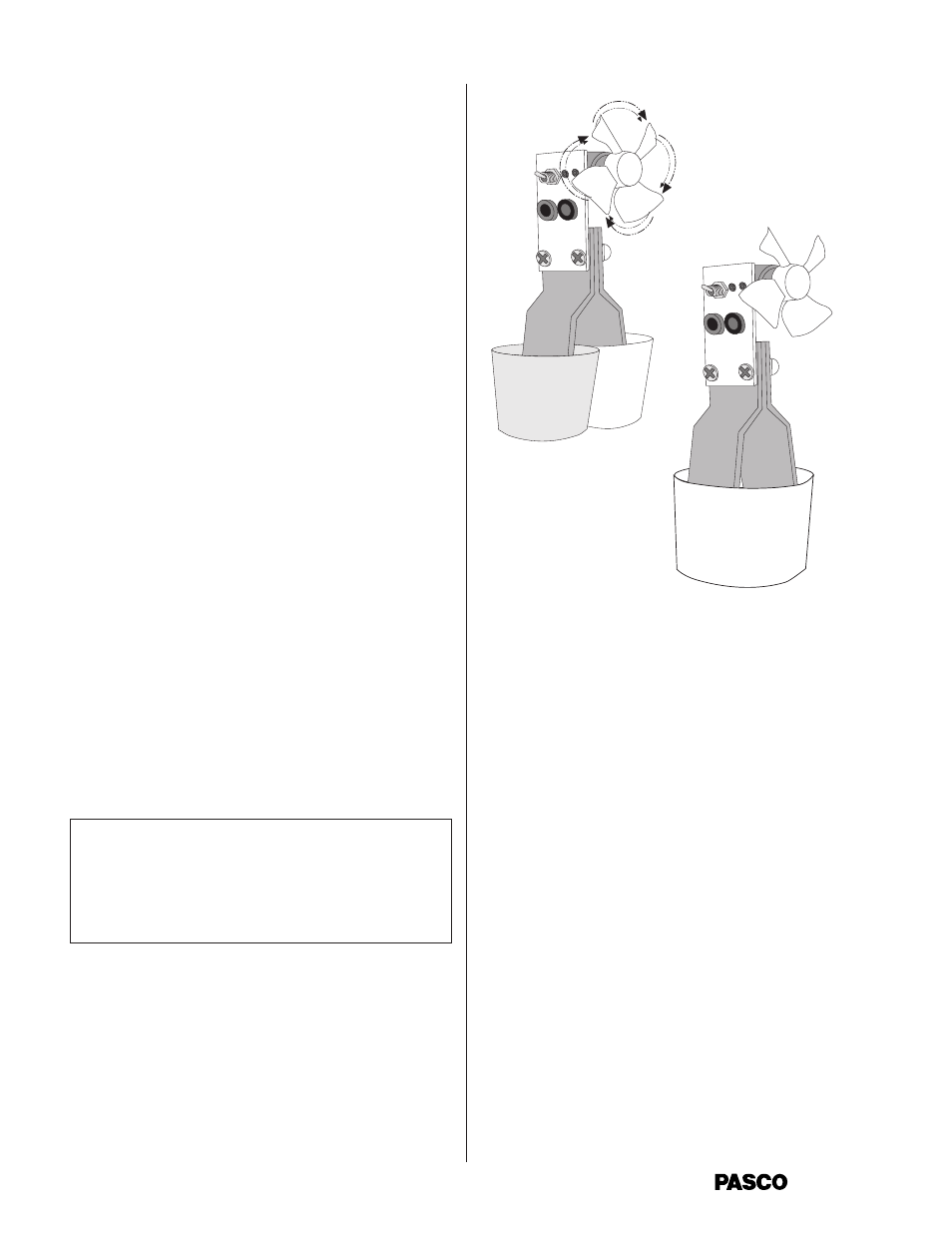
OPERATION
Seebeck Effect:
The PASCO Model TD-8550A Thermoelectric Con-
verter is designed to demonstrate this relationship
between the First and Second Laws of Thermodynam-
ics. The procedure used directly illustrates Kelvin’s
statement of the Second Law. The Converter is used
as illustrated in Figure 2. Put the switch in the “up”
position. One leg of the unit is placed in a cup of cold
water and one in a cup of hot water. (Boiling water and
ice water give good results.) Some of the thermal
energy from the hot water is converted into work by
the Converter, and the fan turns. Then the hot and
cold water are mixed together in a larger container.
Both legs of the unit are placed into the container.
Now the fan does not turn.
The total internal energy of the water is not changed
by mixing the hot and cold together, so there must still
be sufficient energy in the water to turn the fan. But
this would violate the Second Law of Thermodynam-
ics, as stated by Kelvin.
➦
NOTE: As a further demonstration, place
one leg in the mixed water (or in ice water) and
one in a container of dry ice to demonstrate that
there is energy available in the mixed water (and
even in ice water).
This violation of the Second Law can also be ex-
plained in terms of entropy, using the expression
∆
S =
Q/T, where
∆
S is the change in entropy, Q is the heat
transferred, and T is the temperature at which the heat
is transferred. Considering only the heat transfer
taking place in the cups of water, the following holds:
PASCO
scientific
TD-8550A
Thermoelectric
Converter
+
–
A)
∆
T–E
B) E–
∆
T
H
2
O
Th = Tc
FAN DOES
NOT ROTATE
H
2
O
(HOT & COLD MIXED)
PASCO
scientific
TD-8550A
Thermoelectric
Converter
+
–
A)
∆
T–E
B) E–
∆
T
H
2
O
(HOT)
H
2
O
(COLD)
SEEBECK EFFECT
(
∆
T
➞
E)
FAN ROTATES
➃
