İhlas Aura Cebilon Unique User Manual
Page 4
4
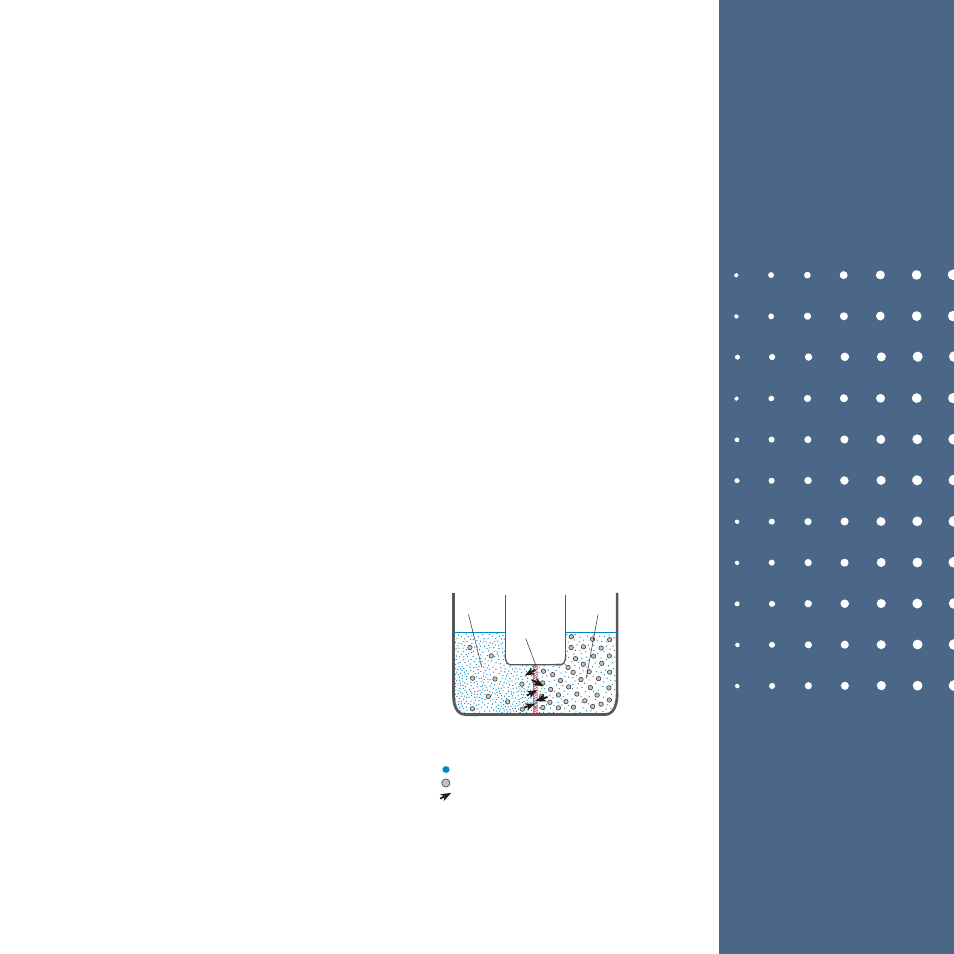
Osmosis, osmotic pressure,
reverse osmosis (ro) process
1
2
3
Before the osmosis
Membrane (semi-permeable)
1-
2-
3-
Low concentration
(high osmotic pressure and high internal energy)
High concentration
(low osmotic pressure and low internal energy)
Solvent (water)
Passage of water molecules through the membrane
Solute (solid particles)
Osmosis is based on a semi-permeable membrane and a
solution; the semi-permeable membrane consisting of a
thin membrane or a thin film allows some molecules or
ions to pass and does not allow some molecules or ions
to pass. Examples of a membrane include dell
membranes and egg membrane.
The solution is a homogeneous blend of more than one
substance. I.e. it is the distribution of a substance in
another substance The solution is a homogeneous
blend of more than one substance. I.e. it is the distribu-
tion of a substance in anot her substance homoge-
neously with small particles invisible to the eye. This
distribution is called dissolution
and the mixture obtained is called solution. Generally
the substance with less amount in the mixture is called
the solute and the substance with more amount is
called the solvent. The best solvent among the many
found in nature is water. Water dissolves many solid,
liquid and gaseous substances. Salt water (sea water)
and sugar water (tea) are well-known solutions. homo-
geneously with small particles invisible to the eye. This
distribution is called dissolution and the mixture
obtained is called solution. Generally the substance
with less amount in the mixture is called the solute and
the substance with more amount is called the solvent.
The best solvent among the many found in nature is
water. Water dissolves many solid, liquid and gaseous
substances. Salt water (sea water) and sugar water (tea)
are well-known solutions.
Many solids exists as dissolved in waters found in
nature. In other words, the water we use is a solution.
The water molecules in this solution continuously on
the move. As the amount of soluble solids increases, the
solid ions occupy the place of the water molecules. In a
water with high concentration i.e. with more solid ratio,
the number of water molecules is less than the water
with same volume but lower concentration, as a result,
since the number of moving molecules is less, the
thermal internal energy will be less as well. I.e., the
energy of the solution with low concentration is higher.
When a semi-permeable membrane is placed between
waters with same volume but different concentrations,
while the water molecules pass through the pores, the
solid particles with a large size cannot pass. Since there
are more water molecules in the water with low concen-
tration and as a result more internal energy, more water
molecules pass across the other side. The pass rate
depends on the concentration ratio, temperature and
pressure. The pass continues until the concentration
ratios in both sides are stabilized.
The water level in the high concentration side increases.
This pressure arising from the potential energy of this
rising water column is stabilized by the Osmotic
Pressure. So, the excess of internal energy in the low
concentration side is stabilized by the potential energy
in the excess of water column in the high concentration
side.
If a pressure equal to the pressure that will be built up
with the excess in the water column is initially applied
to the high concentration side, Osmosis does not occur.
Even, if a pressure more than this amount is applied,
Osmosis is reversed. Despite its small amount, the water
molecules in the high concentration side begins passing
towards the low concentration side. This incident
created by force of pressure is called Reverse Osmosis.
This incident is utilized in Reverse Osmosis Systems to
separate the solid substances dissolved in water. The
purpose of water treatment is to have the water
molecules in the high concentration water (dirty water)
pass to the low concentration side of the water. Osmosis
occurs in the reverse way. In Reverse Osmosis, osmotic
pressure is overcome by applying pressure and thus the
aim is achieved.
