Ionode IJ pH V5.1 User Manual
Page 33
Ionode IJ Instruction Manual
33
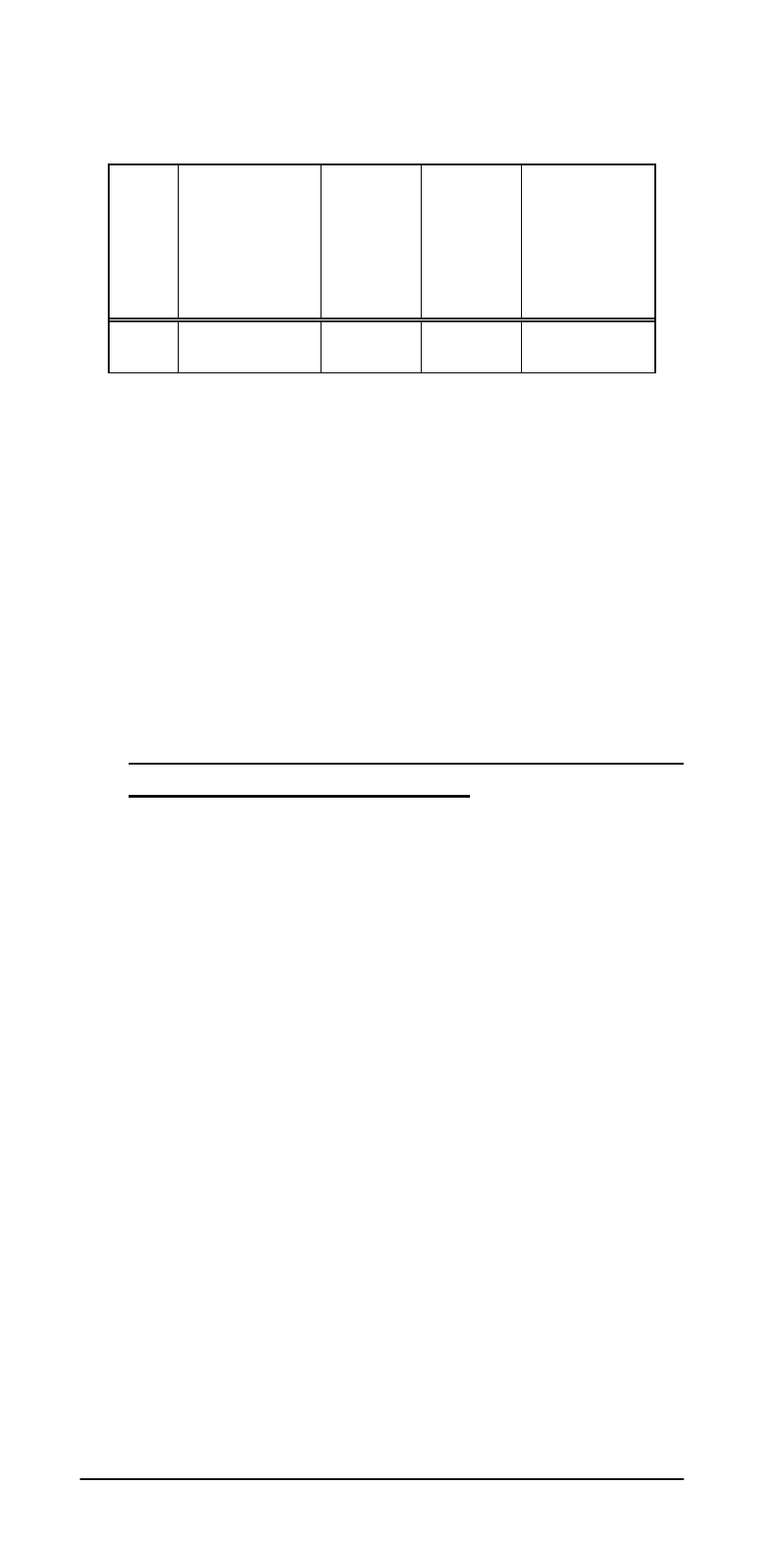
Table 6: Potential Relationships (mV) of
Several Reference Electrodes at 25ºC
S
HE
S
CE
S
atu
rated
KCl
Ag
/Ag
Cl
/
1M
KCl
Ag
/Ag
Cl
/
4M
KCl
Ag
/Ag
Cl
S
atu
rated
KCl
0
+245
+236
+200
+199
For example, a reading of 100mV using an
Ag/AgCl/Saturated KCl reference could be
referred to a SHE reference by adding 199mV.
Although the resistance of an ORP electrode is
very low compared to a pH electrode, it is
preferable to use a high impedance meter in
order to avoid polarising the Ag/AgCl/sat KCl
half cell.
Reference:
2)
“2580 Oxidation Reduction Potential”
Standard Methods for the Examination of
Water
and Wastewater,
19
th
edition,
published by American Public Health Ass.,
American Water Works Ass., and Water
Pollution Control Fed., 1995, 2-73.
