Hanna Instruments HI 83266 User Manual
Page 3
4
5
A major family leisure pursuit is the enjoyment of Swimming Pool and Spa facilities world-wide. A basic necessity
of Pool water treatment, to ensure such enjoyment, is to maintain the water in a safe and pleasant condition
for the bathers.
In order to achieve such an objective, swimming pool water requires testing on daily, and sometimes hourly bases
for disinfection residuals and pH. Equally important, Calcium Hardness and Alkalinity parameters should be
monitored on weekly bases to ensure the pool water is maintained in a balanced condition, thus to avoid system
failure because of corrosion or scale formation.
DISINFECTION RESIDUAL AND pH CONTROL
In terms of swimming pool treatment, disinfection or sanitizing basically means to rid the pool of bather pollution,
destroy bacteria, and control nuisance organisms like algae, which may occur in the pool, filtration equipment,
and piping.
There are a number of techniques used, namely, chlorine, bromine and ozone dosing systems, of which chlorine
is the most common.
Chlorine
Chlorine is a strong oxidizing agent that destroys mostly organic pollutants, bacteria and can combine with
nitrogen containing compounds, forming chloramines. Only a part of the original quantity dosed chlorine,
remains active and continues its disinfecting action.
From the
free chlorine you can distinguish combined chlorine, as that part which combines with nitrogen
containing compound and that is less efficient as a disinfectant. The addition of these two parts gives
total
chlorine. A pool manager needs to aim perfection where free equals total chlorine, and thus to maintain the
combined chlorine concentration near zero. The presence of chloramines is not desired because of the distinctive
‘swimming pool’ smell caused by combined chlorines like di-chloramines. Beside this unpleasant odour it does
irritate the eyes and the mucous membranes.
Commercially chlorine for disinfection may be available as a gas (
Cl
2
), a liquid like sodium hypochlorite or bleach
(
NaOCl) or in a solid state like calcium hypochlorite, chloro-hydantoins or chloro-cyanuric acid compounds. These
compounds, once dissolved in water do establish equilibrium between the hypochlorous acid (
HOCl) and the
hypochlorite ions (OCl¯). Although both forms are considered free chlorine, it is the hypochlorous acid that
provides the strongest disinfecting and oxidising characteristic of chlorine solutions.
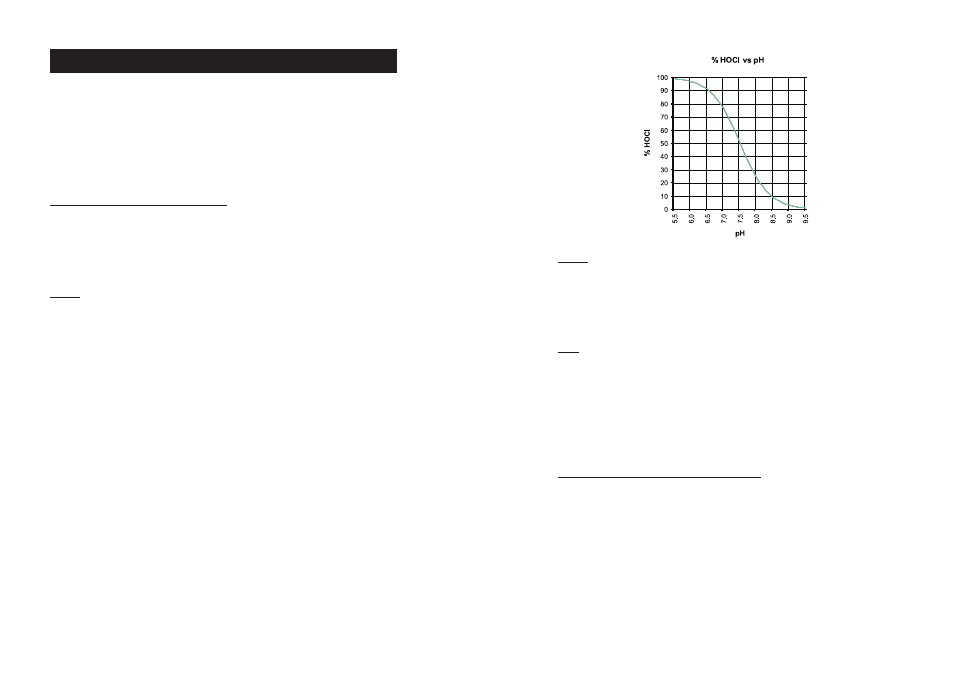
The amount of hypochlorous acid in chlorinated water dependends upon the pH value of the solution. Changes
in pH value will effect the HOCl equilibrium in relation to the hydrogen and hypochlorite ion.
As depicted by the curve on the next page, HOCl decreases and OCl¯ increases as pH increases. At a low pH,
almost all the free chlorine is in the molecular form HOCl and at a pH of around 7.5, the ratio between HOCl and
OCl¯ is 50:50. Since the ionic form OCl¯ is a slow acting sanitizer while the molecular HOCl is a fast acting, it
is important to measure regularly the pH. As a general rule a pH of about 7.2 is recommended to maintain fast
acting disinfection conditions.
SIGNIFICANCE OF POOL AND SPA TESTING
Bromine
In many countries bromine sanitizing has been introduced as an alternative for chlorine, although it is a less
strong sanitizer. The advantage of bromine is its stability at higher temperatures (advantageous for hot well
pools), and its maintained disinfection power at higher pH. Further it does hardly react with nitrogen
compounds, reducing the unpleasant odour,and eye irritation problems. The main disadvantage of bromine is
the slower acting disinfecting power, making it less suitable for larger pools.
Ozone
Ozone is a very strong oxidizing agent that does destroy most difficult to oxidize organic compounds and
chloramines. It thus allows the pool manager to remove very efficiently combined chlorine without refreshing
frequently large amounts of pool water. In general its application is found just before water passes through the
filter units. Its sanitizing power is not pH related.
Mainly because of its strong oxidizing power the return water may contain only trace concentrations of ozone. It
has to be mentioned that ozone is very unstable and there is anyway the need for low-level chlorination to ensure
sanitizing throughout the whole pool.
THE WATER BALANCE AND LANGELIER INDEX (LI)
The pool water characteristics need to be maintained in a balanced condition to avoid system failure. Measuring
the water balance is extremely important to predict if the water is corrosive, scaling or balanced.
A saturation index developed by Dr. Wilfred Langelier is widely used to predict the balance of swimming pool
waters. It is an estimation of the solutions ability to dissolve or precipitate calcium carbonate deposits. A certain
level of this precipitation (filming) is desired to insulate pipes and boilers from contact with water. When no
protective filming is formed, water is considered to be corrosive. On the other hand scaling does cause failure
because of incrustation problems.
In the treatment and monitoring of pool water, the pool manager must ensure that related parameters as
alkalinity, hardness and pH are duly taken into consideration.
