Hanna Instruments HI 83266 User Manual
Page 13
24
25
Calcium Hardness
CALCIUM HARDNESS
SPECIFICATIONS
Range
0 to 500 mg/L (as CaCO
3
)
Resolution
5 mg/L
Accuracy
±10 mg/L ±5% of reading at 25 °C
Typical EMC
±5 mg/L
Deviation
Light Source
Tungsten lamp with narrow band interference filter @ 525 nm
Method
Adaptation of the
Standard Methods for the Examination of Water and Wastewater,
18
th
edition, Calmagite method. The reaction between calcium and reagents causes a
reddish-violet tint in the sample.
REQUIRED REAGENTS
Code
Description
Quantity
HI 93720
A-0
Ca & Mg indicator
0.5 mL
HI 93720
B-0
Alkali solution
0.5 mL
HI 93720
C-0
EGTA solution
1 drop
REAGENT SETS
HI 93720-01 Reagents for 100 tests
HI 93720-03 Reagents for 300 tests
For other accessories see page 46.
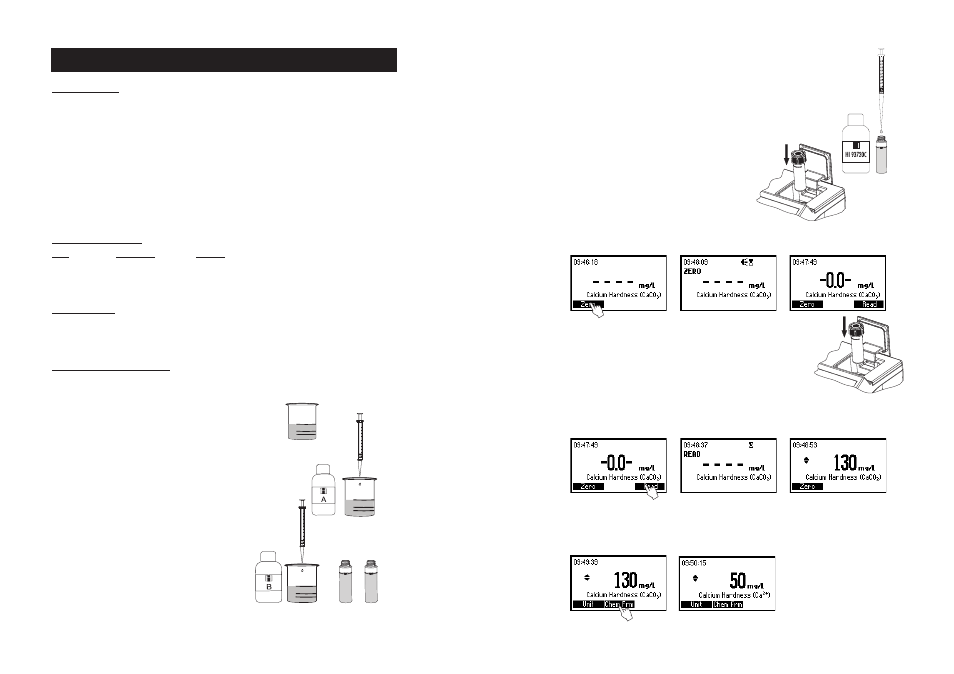
MEASUREMENT PROCEDURE
• Select the
Calcium Hardness method using the procedure
described in the
Method Selection section (see page 15).
• Rinse a graduated beaker several times with deionized
water, fill a 1 mL syringe with the sample, and inject
0.5 mL into the beaker. Fill the beaker up to the 50 mL
mark with hardness-free water.
• Add 0.5 mL of HI 93720A-0 Calcium indicator solution
and swirl to mix.
• Add 0.5 mL of HI 93720B-0 Alkali solution and swirl to
mix. Use this solution to rinse 2 cuvettes before filling
them up to the 10 mL mark.
Calcium Hardness
• Add 1 drop of HI 93720C-0 EGTA solution to one cuvette (# 1), replace
the cap and invert the cuvette several times to mix. This is the blank.
• Place the blank (# 1) into the holder and close the lid.
• Press the
Zero key. The meter will show “-0.0-” when the meter is zeroed and ready for
measurement.
• Remove the blank and insert the second cuvette (# 2) into
the instrument.
• Press
Read to start the reading. The instrument displays concentration in mg/L of calcium hardness,
as CaCO
3
.
• Press ▲ or ▼ to access the second level functions.
• Press the
Chem Frm key to convert the result in mg/L of Calcium (Ca).
# 1
# 2
# 1
# 1
# 2
