Integra LifeSciences Cervical Dilator Set, Reusable User Manual
Page 2
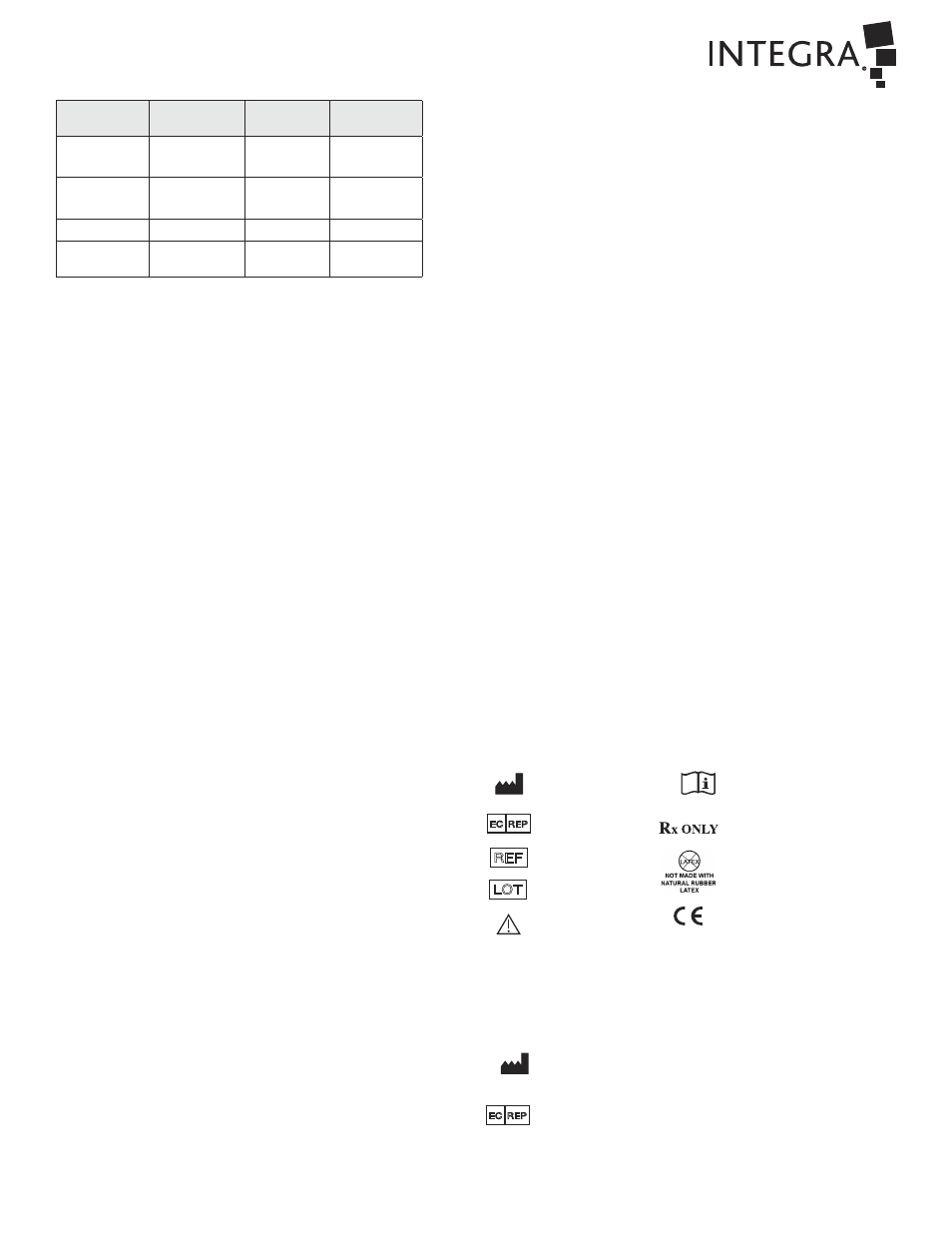
3. Recommended Sterilization Parameters:
Returned Goods Policy
Products must be returned in unopened packages with manufacturer’s seals
intact to be accepted for replacement or credit unless returned due to a complaint
of product defect. Determination of a product defect will be made by Integra.
Products will not be accepted for replacement if they have been in the possession
of the customer for more than 90 days.
Product Information Disclosure
INTEGRA AND ITS SUBSIDIARIES (“INTEGRA”) AND MANUFACTURER EXCLUDE ALL
WARRANTIES, EXCEPT INTEGRA’S APPLICABLE STANDARD WARRANTY WHETHER
EXPRESSED OR IMPLIED, INCLUDING BUT NOT LIMITED TO, ANY IMPLIED
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE.
NEITHER INTEGRA NOR MANUFACTURER SHALL BE LIABLE FOR ANY INCIDENTAL
OR CONSEQUENTIAL LOSS, DAMAGE, OR EXPENSE, DIRECTLY OR INDIRECTLY
ARISING FROM USE OF THIS PRODUCT. NEITHER INTEGRA NOR MANUFACTURER
ASSUME NOR AUTHORIZE ANY PERSON TO ASSUME FOR THEM ANY OTHER
OR ADDITIONAL LIABILITY OR RESPONSIBILITY IN CONNECTION WITH THESE
PRODUCTS.
Sterilizer
Exposure
Temperature
Exposure
Time
Minimum
Dry Time
Pre-Vacuum
(Wrapped)
132°C (270°F)
4 minutes
20 minutes
Pre-Vacuum
(Un-wrapped)
132°C (270°F)
4 minutes
-
Gravity Steam
121°C (250°F)
30 minutes
30 minutes
Gravity Steam
132°C ~ 135°C
(270°F ~ 275°F)
10 minutes
30 minutes
Consult Instruction for Use
CAUTION: Federal (USA) law
restricts this device to sale by or
on the order of a physician
Not made with natural
rubber latex
Product complies with
requirements of directive 93/42/
EEC for medical devices
Manufacturer
1
European Authorized
Representative
Catalog number
Lot Number
Caution! See Warnings
and Precautions
1
Company responsible for a device marketed under its own name regardless of
whether “manufactured for” or “manufactured by” the company.
EC REP
REF
LOT
EU Representative
Wellkang Ltd
i
Suite B, 29 Harley Street
i
LONDON, W1G 9QR, U.K.
Manufactured for
Integra York PA, Inc.
i
589 Davies Drive, York, PA 17402
866-854-8300 USA
i
+1 717-840-2763 outside USA
i
+1 717-840-9347 fax
integralife.com/integra-miltex
Integra, the Integra logo, and Miltex are registered trademarks of Integra LifeSciences Corporation
or its subsidiaries in the United States and/or other countries. All other trademarks are property
of their respective owners. ©2012 Integra LifeSciences Corporation. All Rights Reserved.
SURGOSDILSETDFU Rev. - 04/13
Manufacturer
Panpac Medical Corporation
i
6F-2, No. 202, Sec. 3, Ta-Tong Rd.,
Shi-Chih Dist., New Taipei City
i
TAIWAN, R.O.C.
i
Phone: 886-2-8647-2242
Fax: (886) 28647-2770
EC REP
Symbols used on labeling
0434
