Cell line data – Leica Biosystems Bond Oracle HER2 IHC System User Manual
Page 14
English
Page 14 of 23
English
Leica Biosystems Bond Oracle HER2 IHC System Instructions for Use TA9145 EN-CE-Rev_E 18/06/2013
Cell Line Data
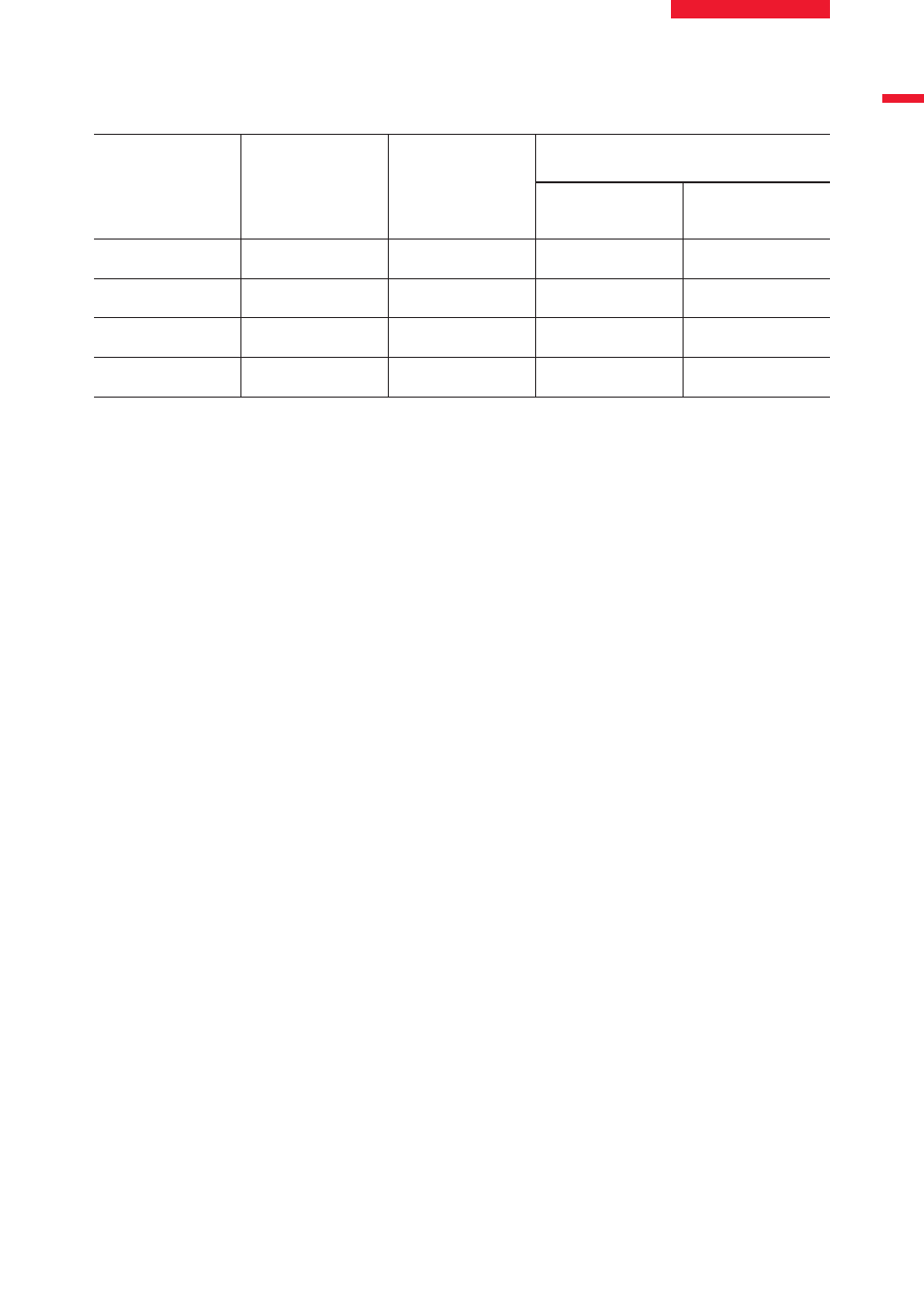
Cell Line
BOND Oracle
HER2 IHC System
Profile
HER2
Receptor Load
per Cell*
HER2 Gene Amplification Status
+
HER2 Copy
Number
HER2:Chr17
Gene Ratio
SK-BR-3
3+
4.3x10
5
13.35
3.55
MDA-MB-453
2+
1.4x10
5
5.73
2.05
MDA-MB-175
1+
6.3x10
4
3.33
1.20
MDA-MB-231
0
9.3x10
3
3.15
1.13
*HER2 receptor load analysis as assessed by flow cytometry.
+
HER2 Gene Amplification Status as assessed by dual probe
(HER2:Chromosome 17) FISH.
Table 5. HER2 Control Slide profile
Clinical Concordance of Bond Oracle HER2 IHC System v Dako
HercepTest
Part one of the study examined the suitability of the Bond Oracle HER2 IHC System for use
as an aid in determination of treatment with Herceptin® (trastuzumab) therapy. The study was
designed to examine the concordance between the Bond Oracle HER2 IHC System and the
Dako HercepTest, considered as the ‘gold standard’ for this assay. The acceptance criterion
was defined as greater than 75% overall concordance between the two tests with a 95%
confidence interval (CI).
The study was conducted as a two-site, US based, blinded evaluation. Each investigational site
was supplied with formalin-fixed, paraffin-embedded breast cancer samples of known HER2
status. Cases were selected in reverse consecutive order from the clinical archives, representing
the consecutive flow of cases into a histopathology department for clinical testing, and tested
independently of other prognostic and/or predictive factors, with no bias introduced to the cohort.
Cohorts of 160 and 292 specimens were tested at Site 1 and Site 2 respectively. Each cohort
had an equal representation of equivocal/positive (2+, 3+) and negative (0, 1+) cases, based on
previously assigned HER2 IHC scores, resulting in a total study population of 452 samples.
Twelve samples were considered unsuitable, due to lack of sufficient invasive tumor and were
removed from the study. A further nine samples could not be scored as a result of tissue lifting
from the slide surface, resulting in a final study population of 431 samples.
All cases were stained with the HercepTest according to the manufacturer’s instructions as
specified in the package insert. Sequential sections from each case were stained with the Bond
Oracle HER2 IHC System on board an automated Leica Biosystems BOND fully automated,
advanced staining system. All cases were de-linked from unique patient identifying information
and were accompanied by clinical data relating to tumor size, tumor stage, tumor grade and
estrogen receptor status.
All stained slides were masked and scored in a randomized fashion by trained observers at
two sites. For 2x2 concordance analysis, scores were interpreted as negative if the staining
intensity was 0 or 1+, and positive for scores of 2+ or 3+. For 3x3 concordance analysis, scores
were interpreted as negative if the staining was 0 or 1+, equivocal for scores of 2+ and positive
for scores of 3+. Data was then analyzed for positive staining agreement and negative staining
agreement.
