Merit Medical PD Percutaneous Kit IFU User Manual
Percutaneous pd catheter implantation system
IMPLANTATION STENCIL
Place on Patient’s
Cranial Border of
the Pubic Symphysis
For directions, see
Instructions for Use
Use with Flex-Neck
®
Classic & Arc™ Adult
PD Catheter ONLY
Classic Exit
Cuff Site
Pr
imar
y I
ncision Sit
e
Rec
tus C
uff Sit
e
Flex-Neck
®
Adult PD Catheter
PERCUTANEOUS
PD CATHETER
IMPLANTATION SYSTEM
I N S T R U C T I O N S F O R U S E
VP – 511 and VP-511M
Implantation System for Peritoneal Dialysis Catheters
Product Description:
Implantation System Components:
• 0.038” Guide Wire
• 12 French Dilator
• 14 French Dilator
• 18 Gauge Introducer Needle
• 18 French Peelable Introducer Sheath
• Cuff Implantor™
• Faller Trocar
• Scalpel
• 10 mL Syringe
• 4x4 Gauze
• Clip
Indications for Use:
The Percutaneous Implantation Kit can be used to implant
a peritoneal dialysis catheter in patients who are suitable
candidates for peritoneal dialysis therapy.
Contraindications:
• Do NOT use if the patient is not a suitable candidate for
peritoneal dialysis therapy.
Px Only: Caution: Federal (USA) law restricts this device to
sale by or on the order of a physician.
Precautions:
• Read manufacturer’s instructions prior to use.
• Contents are sterile (via ethylene oxide). Do not use if
packaging is opened, damaged, or broken.
• For single patient use only. Do not reuse, reprocess, or
resterilize. Reuse, reprocessing, or resterilization may
compromise the structural integrity of the device and/
or lead to device failure, which in turn may result in
patient injury, illness, or death. Reuse, reprocessing, or
resterilization may also create a risk of contamination
of the device and/or cause patient infection or cross
infection, including, but not limited to, the transmission
of infectious disease(s) from one patient to another.
Contamination of the device may lead to injury, illness,
or death of the patient.
• Do not use after expiration date.
• The medical techniques, procedures, and potential
complications stated herein do NOT give full and/
or complete coverage or descriptions. They are not a
substitute for adequate training and sound medical
judgment by a physician.
• Use an aseptic procedure to open the package and to
remove the contents.
Potential Complications:
Peritoneal Dialysis catheter implantation procedures have
inherent risks associated with their use. All such risks
apply to the use of the Percutaneous Implantation System.
Peritoneal dialysis potentially has a number of complica-
tions that may occur, which generally are not caused by
the implantation, but may affect the quality of therapy.
These complications may include, but are not limited to,
the following:
• Infections (exit-site or tunnel)
• Peritonitis
• Sepsis
• Bowel perforation
• Leakage (initial or latent)
• Fluid flow obstruction (inflow or outflow)
• Bleeding (subcutaneous or peritoneal)
• Ileus
• Proximal exit cuff erosion
• Distal (rectus/deep) cuff erosion
• Risks normally associated with peritoneoscopic and
laparoscopic procedures
• Allergic reaction
• Abdominal pain
• Infusion pressure/pain
• Organ erosion
• Genital edema
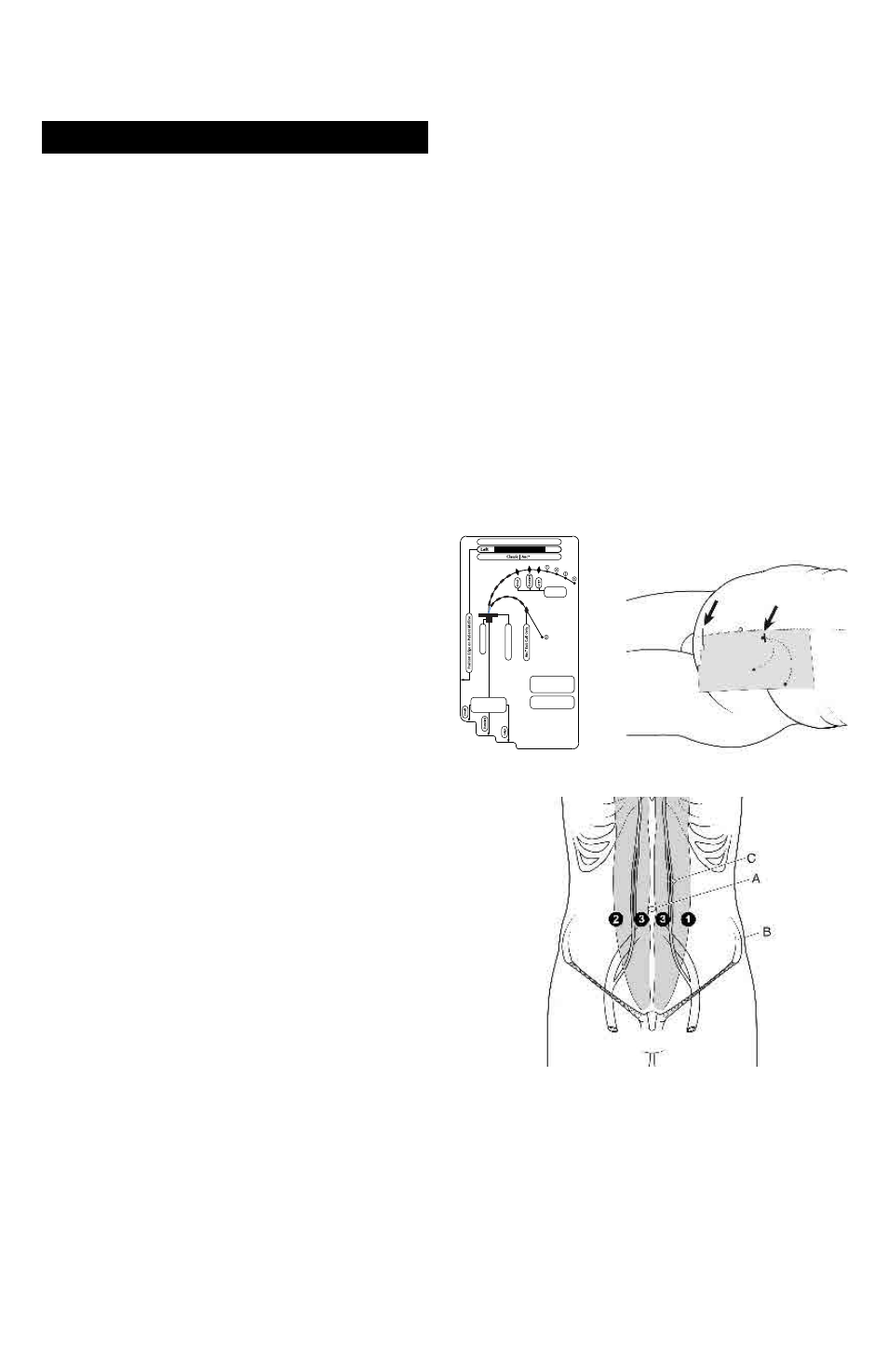
Catheter Implantation Site Options
An Implantation Stencil may help to achieve consistent ef-
fective catheter placement and assure proper coil location.
Implantation Stencils (Figure 1) are sold separately with the
Flex-Neck® Catheter kits.
PD Catheter Implantation Site Options
Locate preferred implantation, tunnel, and exit sites as
indicated by an appropriate Implantation Stencil (Figure 2).
Please see anatomical landmarks as indicated in Figure 3.
Figure 1
Figure 2-Stencil on body
Implantation Stencil
Figure 3 – Potential lower catheter implantation sites
A. Umbilicus
B. Iliac crest
C. Inferior and superior epigastric arteries
1. Left, lateral border of rectus sheath, 2-3 cm below
umbilicus
2. Right, lateral border of rectus sheath, 2-3 cm below
umbilicus
3. Medial border of rectus sheath, 2-3 cm below umbilicus
NOTE: Implantation sites should be above superior iliac
crest.
