PASCO CI-6535 RESPIRATION RATE SENSOR User Manual
Page 5
012-07034A
Respiration Rate Sensor
5
Lift up on the top flap of the respiration belt to
disengage the hook-and-pile strips from each other
when you want to remove the belt.
Suggested Experiments
Respiration Rate versus Activity
Monitor respiration rate before and after exercise.
Measure the respiration rate while resting. Then
exercise vigorously. Measure the respiration rate
immediately after exercise, and the measure how long
it takes for the respiration rate to return to the resting
(“normal”) rate.
Respiration rate (number of breaths per unit of time)
depends on several factors: altitude, lung capacity,
health, and level of activity. Higher altitudes and levels
of activity would tend to increase respiration rate.
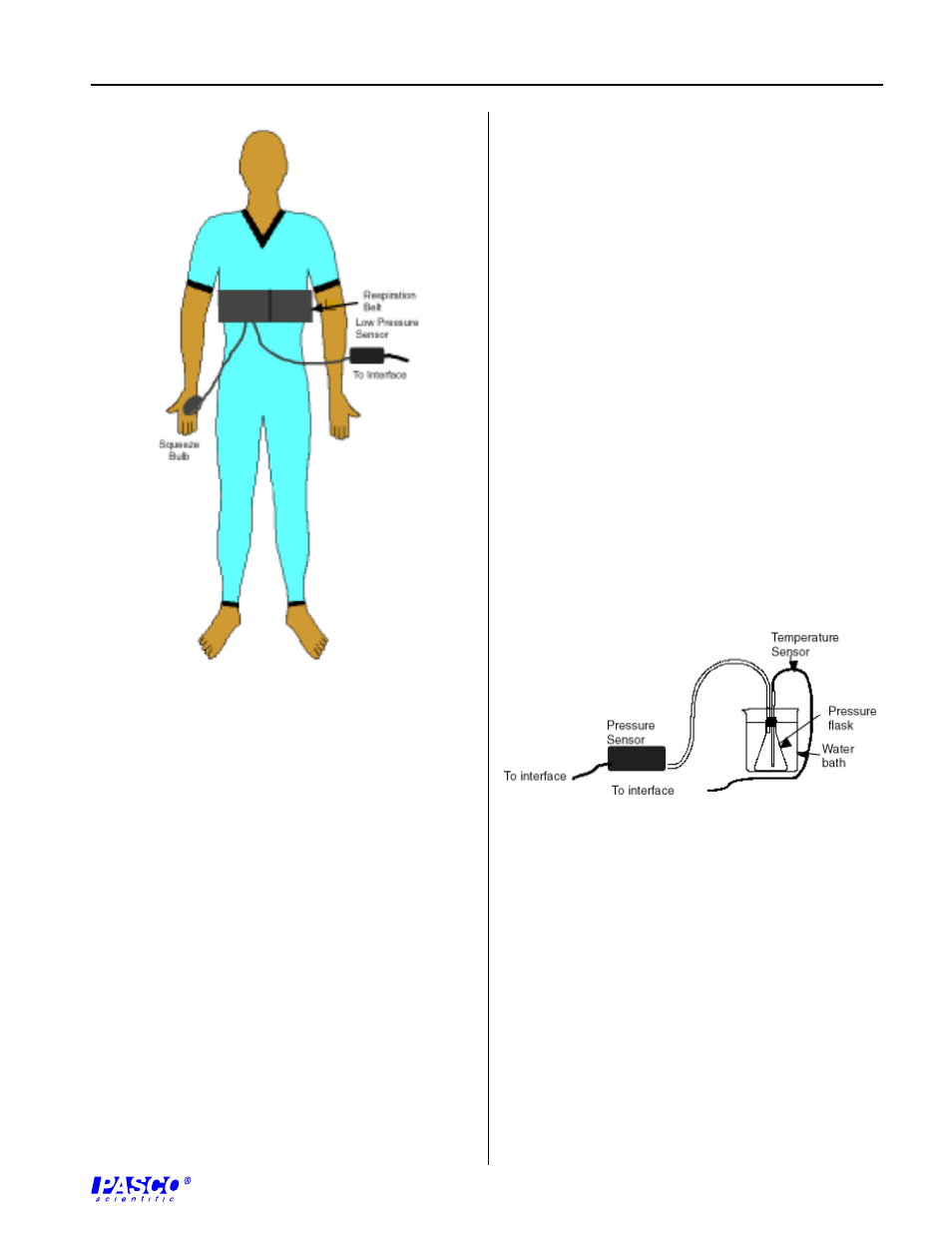
Figure 7
Respiration Belt In Place
Larger lung capacity and generally good health would
tend to decrease respiration rate.
Gay-Lussac’s Law (pressure vs. absolute
temperature)
Gay-Lussac’s Law states that if the volume remains
constant, the pressure of a container of gas is directly
proportional to its absolute temperature. Set up a
sealed container of air by attaching the longer piece of
plastic tubing to a stopper in a 125 mL Erlenmeyer
flask. Put a drop of glycerin on the bottom of one hole
of a two-hole rubber stopper. Put the glass part of an
eyedropper tip end up through one hole in the rubber
stopper. CAREFULLY put the end of the plastic
tubing over the tip of the eyedropper. Connect the
other end of the tube to the pressure port connector at
the front of the pressure sensor unit.. Put a drop of
glycerin on the top of the other hole. Insert a
temperature sensor through the hole. Place the stopper
in the top of the flask. See Figure 8.
Place the flask in water baths of different
temperatures. Record data on how the pressure
changes with the temperature changes.
Pressure in Liquids
Put the end of the longer piece of tubing under water.
The pressure reading should increase by 0.0978 kPa
(0.02896 in of mercury) per centimeter of depth below
the surface. You can also use a “J” shaped tube to
study how pressure relates to the difference in heights
of the liquid in the two parts of the tube.
Figure 8
Experiment Setup For Gay-Lussac’s Law
