Aqua-Pure APIF100 User Manual
Page 5
SECTION 2: BEFORE INSTALLATION
Inspecting And Handling Your Filter:
Inspect the equipment for shipping damage. If damaged, notify the transportation company and request a damage inspection. Handle the fi lter with care. Damage
can occur if dropped or set on sharp, uneven projections on the fl oor. Do not turn the fi lter upside down. Installation must comply with state and local laws and
regulations.
Make Sure Your Water Has Been Thoroughly Tested:
An analysis of your water should be made prior to the selection of your water conditioning equipment. Your dealer will generally perform this service for you,
and may send a sample to the factory for analysis and recommendations. Enter your analysis below for your permanent record.
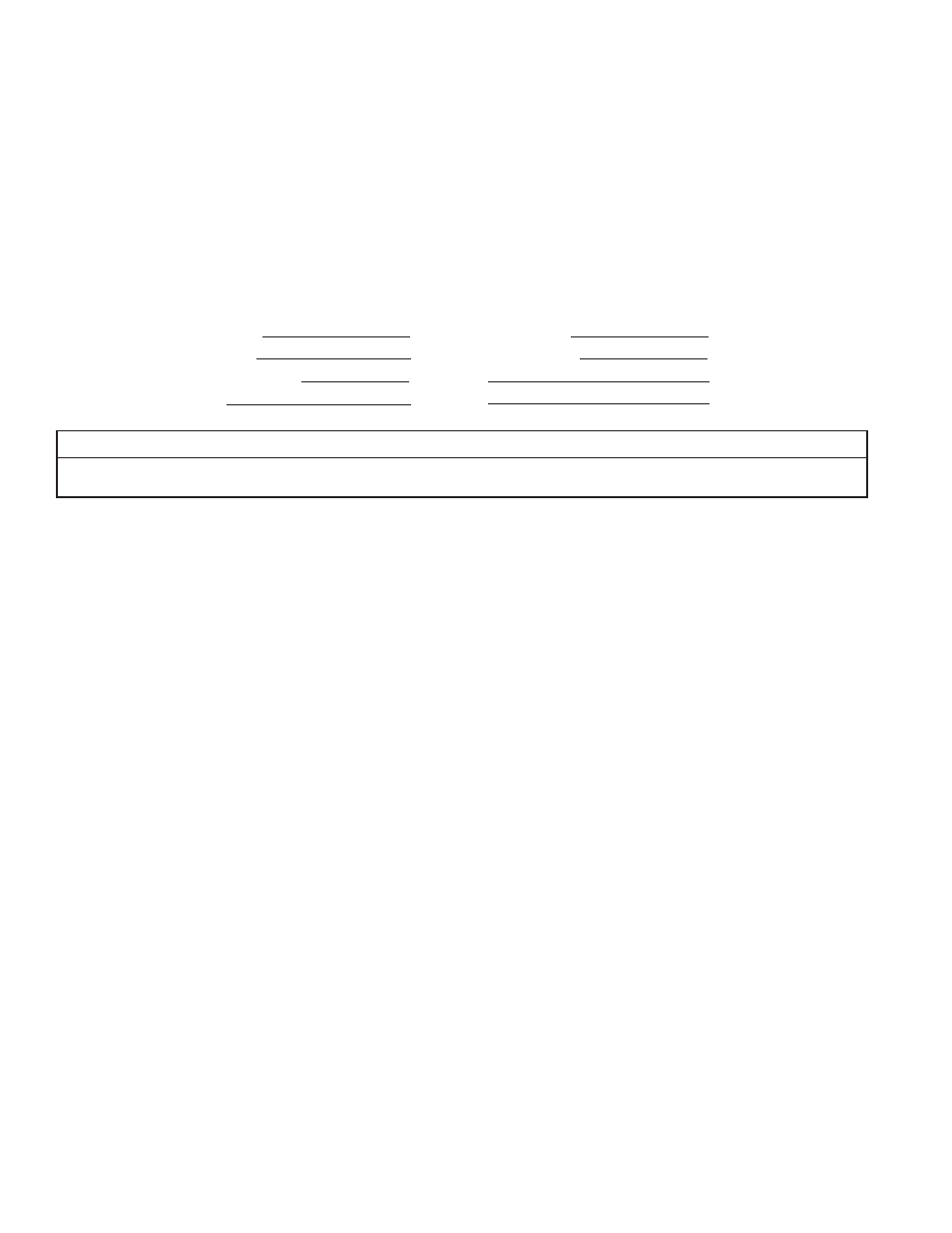
Analysis of Your Water:
Hardness gpg
Tannins (Humic Acid) ppm
Iron (Fe) ppm
Hydrogen Sulfi de (H
2
S) ppm
Manganese (Mn) ppm
Other ppm
pH ppm
Other ppm
IMPORTANT NOTES
IMPORTANT NOTES
Hydrogen sulfi de (H
2
S) must be tested for at the well site. For accuracy, the sample must be drawn with the pump RUNNING, and the test be completed
within ONE minute after the sample is drawn.
Iron (Fe)
Iron concentrations as low as 0.3 ppm (0.1 ppm under some conditions) will cause staining. The iron concentration, together with the fl ow rate demand and the
consumption rate of the water determines the size of the fi lter system required. The higher these factors are, the larger the required system. The iron and manga-
nese reduction system is capable of reducing the three main types of iron found in water supplies: dissolved iron; precipitated iron; and bacterial iron. There is an
upper limit of 15 ppm iron concentration for the iron and manganese reduction system; special care must be taken when selecting a fi lter model if your water has
a combination of high iron, very low pH and/or manganese levels above 0.2 ppm.
The iron and manganese reduction system is not bactericidal, i.e. it does not remove or kill “bacterial iron”. It reduces the iron upon which the bacteria may live or
which it deposits in your plumbing fi xtures, thus helping to minimize its effects.
Manganese (Mn)
The presence of manganese can be bothersome, even for an iron and manganese reduction system (and is problematic for chemical oxidizing systems because the
chemicals may not allow for the correct pH for Manganese Reduction). As little as 0.05 ppm of manganese can produce a brownish or black stain. The ability of the
iron and manganese reduction system to reduce manganese depends on its concentration and the pH of the water.
The oxidation of manganese is very similar to that of iron, therefore, a pH of 8.2 or higher must be obtained. When this pH level is achieved, the precipitation of
manganese may more readily occur. To accomplish this, models are available where the media contains additional quantities of pH Plus, the pH raising component
(model designations with “M” suffi x). In any application involving manganese, a larger model fi lter is generally recommended (but only if the pumping rate is suf-
fi cient to backwash the larger size).
If, however, the manganese concentration is low (0.1 ppm or less) and the pH is 6.5 or higher, an iron and manganese reduction system containing standard iron and
manganese reduction system media will generally perform satisfactorily, although backwashing should be performed at more frequent intervals. Under more severe
conditions where the pH is very low and/or the manganese concentration is high, an acid neutralizer installed ahead of the iron and manganese reduction system
will maintain the required 8.2 pH level longer than the chem-free media.
pH
The pH of water measures the hydrogen ion concentration. Water with a pH of less than 7.0 is base, above 7.0 it is alkaline, and a pH of 7.0 is neutral. The lower
the pH value, the greater the acidity, and the higher the pH value, the more base. Acidic water (pH less than 7.0) is corrosive to pipes, appliances, etc. A pH of 7.0 or
higher facilitates iron reduction, which is why the iron and manganese reduction system is designed to increase the pH when it is less than 7.0.
The pH increasing component of chem-free media is “sacrifi cial”, that is, it slowly dissolves during the process of increasing pH. The rate at which this occurs is
proportional to the degree of the pH increase and the water consumption rate (i.e., the greater the pH increase and water consumption, the greater the sacrifi cial
rate). Thus, when the pH is increased to 8.2 or more, as is necessary when manganese is present, the sacrifi cial rate is even greater. Under the most severe condi-
tions, the pH Plus component of the media may have to be replenished two to four times per year. On the other hand, if the raw water pH is 7.0 or above and no
manganese is present, the sacrifi cial rate is very slight (see IMPORTANT NOTE, Section 1).
2-1
