Precipitation demonstration, Test procedure – LaMotte AT Visual Series Water Softener Demo Kit User Manual
Page 12
12
Precipitation
Demonstration
Again, Calcium and Magnesium ions are the major contributors to water hardness. The chemical reagents in this
demonstration pull the Calcium and Magnesium ions out of solution to form a cloudy precipitate in hard water.
The water that has been run through the ion exchange column has had these ions removed, therefore, the sample
should remain clear.
NOTE: This portion of the AT-38/40 Water Quality Demo Kit is ONLY a visual demonstration illustrating the
removal of Calcium and Magnesium ions from tap water after treatment by the ion exchange process. The
results should not be interpreted beyond the intent of the demonstration.
*WARNING: Reagents marked with an * are considered to be potential health hazards. To view or print a Mate-
rial Safety Data Sheet (MSDS) for these reagents go to www.lamotte.com. To obtain a printed copy, contact
LaMotte by e-mail, phone or fax.
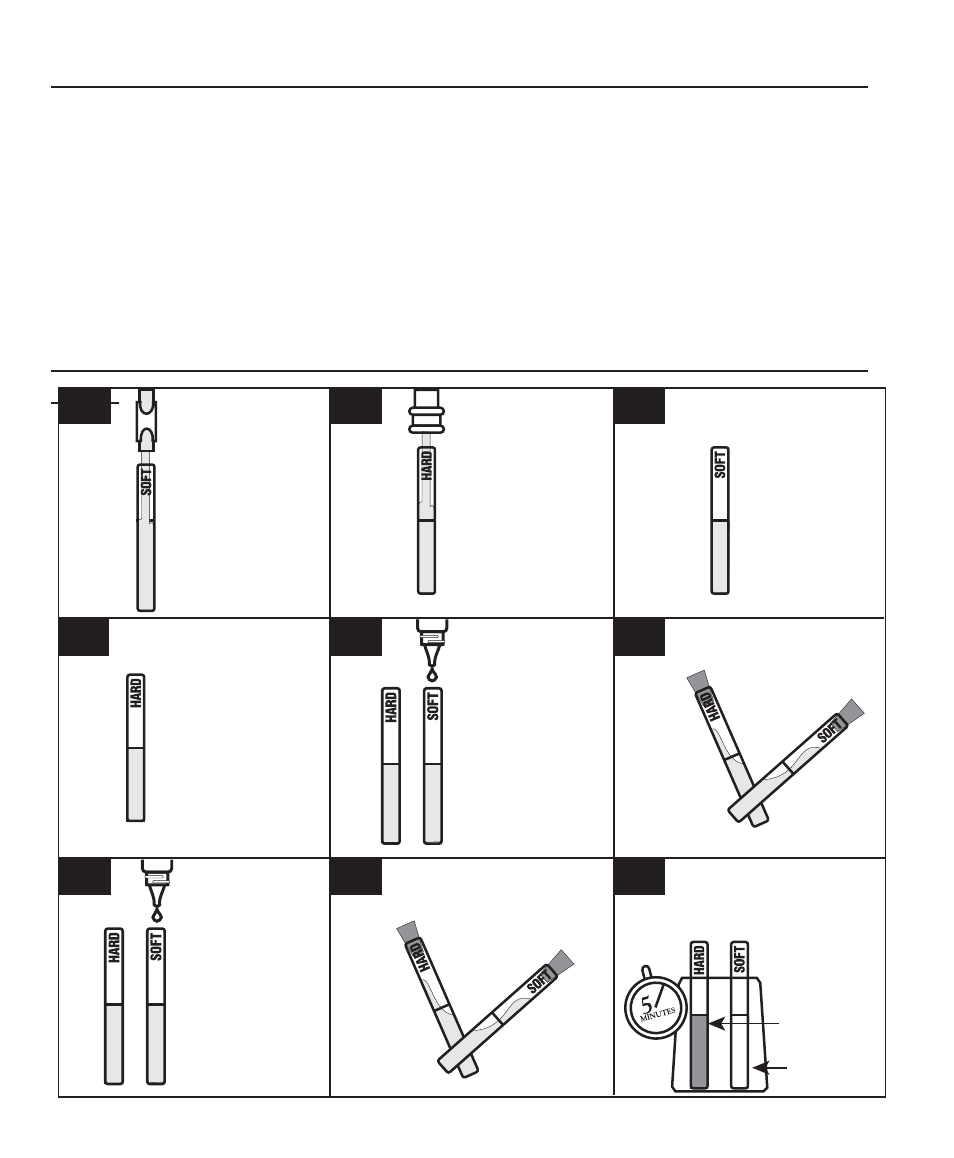
TEST
PROCEDURE
Thoroughly rinse the
“SOFT”
water Demo Tube
(0298) with
softened water.
Thoroughly rinse the
“HARD”
water Demo Tube
(0297) with
untreated water.
Fill the “SOFT”
Demo tube (0298)
to the line with
softened water.
Fill the “HARD”
Demo tube (0297)
to the line with
untreated water.
Add 7 drops of
*Precipitation
Reagent A
(4542WT)
to each tube.
Cap and mix.
Add 7 drops of
*Precipitation
Reagent B
(4543WT)
to each tube.
Cap and mix.
Place tubes in the
Precipitation Rack (0879)
and allow the tubes to
stand for
5 minutes.
Heavy
Precipitate
Remains
clear
1.
2.
3.
4.
5.
6.
7.
9.
8.
