Ombustion, Asics – UEi Test Instruments C127OILKIT User Manual
Page 12
i
NtroduCtioN
UEi combustion analyzers provide real time data that provides information on the condition of the combus-
tion process of your equipment. This information is needed for proper setup and maintenance to verify proper opera-
tion. Benefits of combustion analysis are to increase efficiency thus reducing fuel costs, verification of proper combus-
tion to reduce future problems, and to check for safe operation. A combustion process out of balance can increase
maintenance needs, create excess emissions, lead to safety concerns or waste fuel and money. By checking for proper
operation you are able to confirm a job well done.
• This overview will explain some of the common terms used in combustion testing
– The combustion process – (a small amount of chemistry)
– Ideal combustion – you may have heard the term Stoichiometric (or not)
– Relationship between CO2, CO and O2
• The analyzer and display
– Various text and icons used on the front housing, in the display and on the printout
– What will the readings do when adjustments are made
– Where are you on the combustion curves from these readings
t
he
C
ombuStioN
P
roCeSS
What is really going on during combustion? Most of us know it as a fire that is generating heat and possibly
smoke. We know that paper or wood can burn when lit, and continue to burn – but what is really happening?
Combustion is a continuous chemical reaction that occurs when a certain temperature is reached, and there is the
presence of both fuel and an oxidizer. The most common fuels are hydrogen and carbon, and the typical oxidizer is
O2 present in the air we breathe. Once the reaction is started it will continue as long as it is being fed fuel and oxy-
gen, and the temperature is sufficient.
i
deal
C
ombuStioN
P
roCeSS
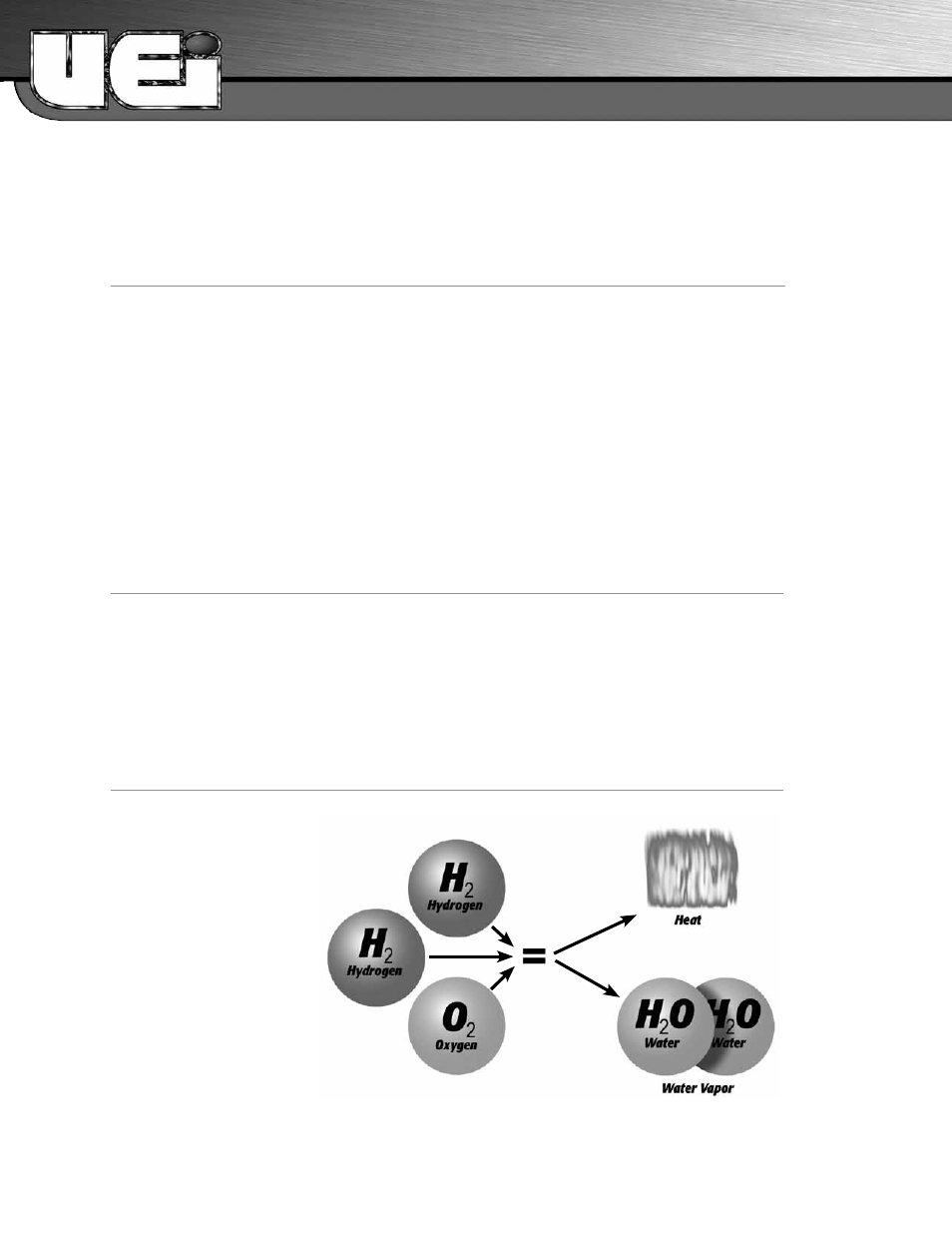
If a perfect condition
could exist for combustion it would
be the burning of pure hydrogen
(H2) in pure oxygen (O2). This
would give us heat and water, and
be the easiest to maintain. Two
hydrogen (H2), combine with one
O2 molecule gives us two water
molecules plus heat. The reaction
would be something like figure 1.
It is great in theory that we would
have a very efficient system with
only some heat losses from the
water vapor, but it isn’t very prac-
tical. Pure hydrogen and oxygen
are expensive to create, and diffi-
cult to handle compared to other
fuels already available.
C
OMBUSTION
B
ASICS
figure 1
12
13
