Thermo Fisher Scientific Ion Selective Electrodes Copper User Manual
Page 17
Instruction Manual
Copper Electrode
17
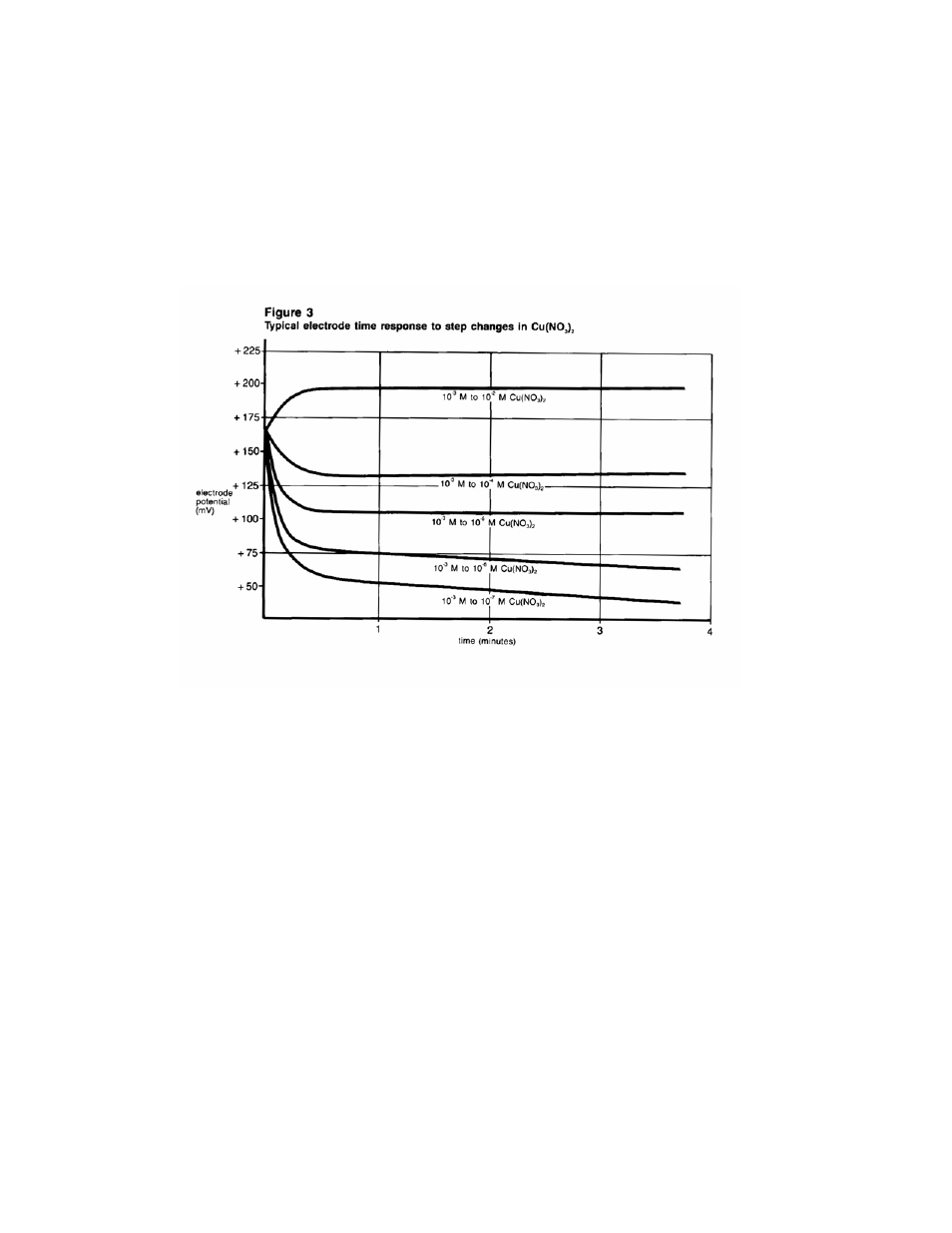
The time needed to reach 99% of the stable electrode potential
reading, the electrode response time, varies from several seconds
in highly concentrated solutions to several minutes near
concentrations of 1.0X10
-5
M cupric ion. Below 10
-5
M, considerably
longer response time can be expected. See Figure 3.
A drifting potential reading or a decrease in electrode slope may
mean that the electrode membrane needs polishing.
To polish the membrane:
1.
If using polishing paper, cut off a 1-2" piece and
place it face up on the lab bench.
2.
Put a few drops of distilled or deionized water in the
center of the paper.
3.
Holding the paper (cotton) steady with one hand, bring
the membrane of the electrode down perpendicular to the
paper and, with a slight swirling motion, gently polish
the tip of the electrode against the surface of the
polishing paper (cotton) for a few seconds.
4.
Rinse the electrode surface with distilled or deionized
water and soak the electrode tip in standard solution
for about five minutes before use.
5.
If using jeweller's rouge, place a cotton ball on the
