KROHNE CORIMASS MFC 85 EN User Manual
Page 9
9
4.
General Concentration
4.1
Mixtures Of Two Immiscible Non Compressible Components
Immiscible means the two components do not mix or interact with each other. Typical
examples are oil/water emulsions or solid/liquid suspensions. For these cases, if you have V
S
volume of one component and V
C
of the other, then on mixing, the total volume V
T
is :
V
T
= V
S
+ V
C
Also for the masses :
M
T
= M
S
+ M
C
Also the densities of the components
ρ
S
,
ρ
C
and the mixture
ρ
M
are related by :
ρ
S
S
S
M
V
=
,
ρ
C
C
C
M
V
=
and
ρ
M
T
T
M
V
=
It can be shown that :
C
V
M
C
S
C
=
−
−
×
ρ
ρ
ρ
ρ
100%
Equation 1
C
M
S
M
M
C
S
C
=
⋅
−
−
×
ρ
ρ
ρ
ρ
ρ
ρ
100%
Equation 2
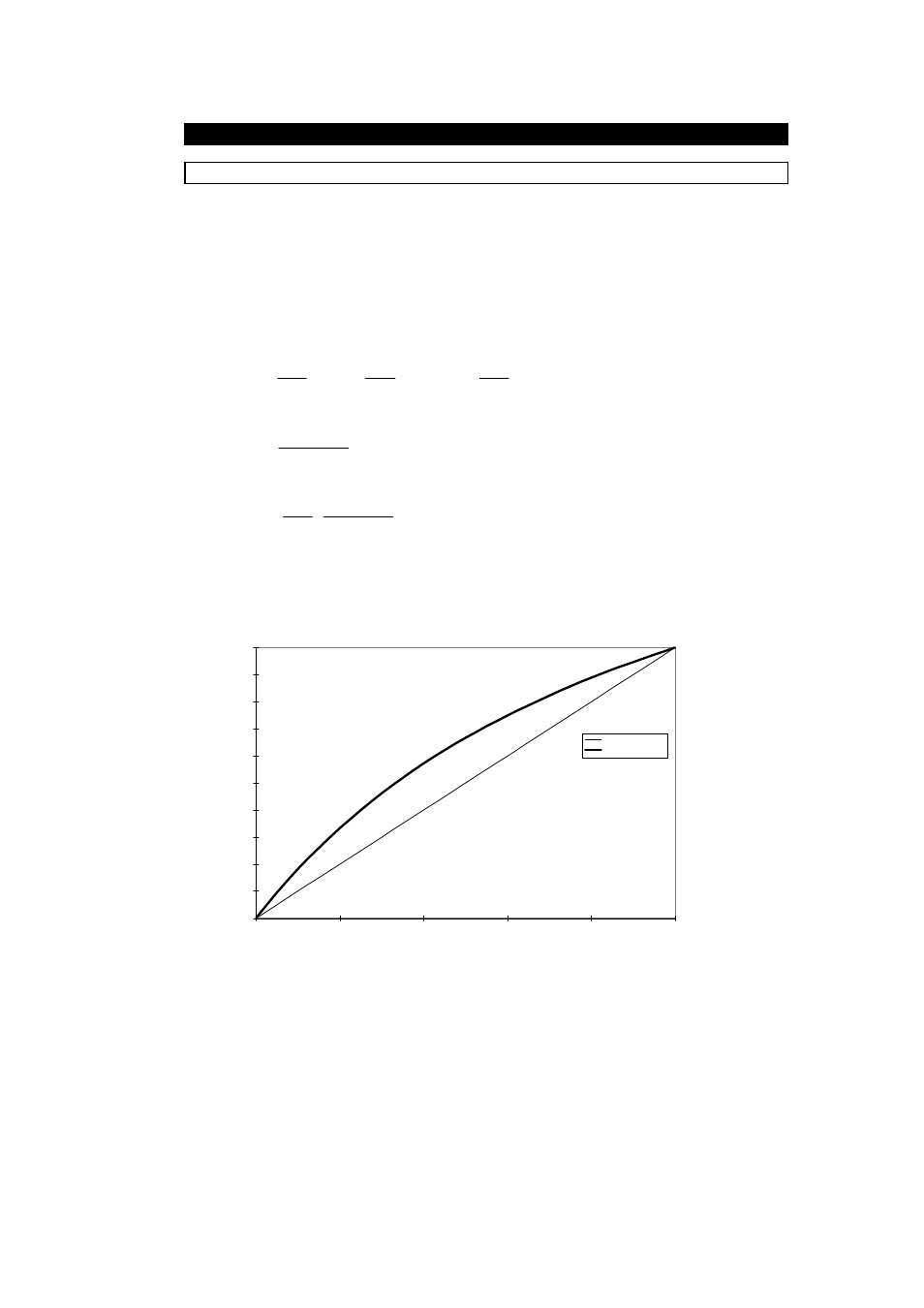
The figure below shows graphically these two equations for the case
ρ
S
=2.0 and
ρ
C
=1.0.
As can be seen, the C
V
curve is linear whilst the C
M
curve is not.
Concentration curves for Immiscible Mixtures
0.00%
10.00%
20.00%
30.00%
40.00%
50.00%
60.00%
70.00%
80.00%
90.00%
100.00%
1.000
1.200
1.400
1.600
1.800
2.000
Density (g/cc)
Conc. by Volume
Conc. by Mass
Ps = 2.0g/cm3
Pc = 1.0g/cm3
It is these two equations that are used in the MFC081/085’s general concentration algorithm.
ρ
M
is measured directly by the meter and
ρ
S
and
ρ
C
are known for the components in question.
