Ordering information, Models, Optional accessories – Atec Fluke-451B User Manual
Page 3: Fluke biomedical
Ordering
Information
Models
451B-RYR Ion Chamber
Survey Meter with Beta Slide
and standard chamber
451B-RYR-SS Ion chamber
Survey Meter with molded grip
handle and shoulder strap
Optional accessories
451EXL 451 Assistant for
Excel, includes RS-232
interface cable
190HPS Single Unit
Carrying Case
62-103 Check Source,
137
Cs, 10 µCi. Flat disc,
1-inch diameter
450UCS Check Source,
238
Uranium, 0.064 µCi,
impregnated, 2 in x 2 in
yellow card
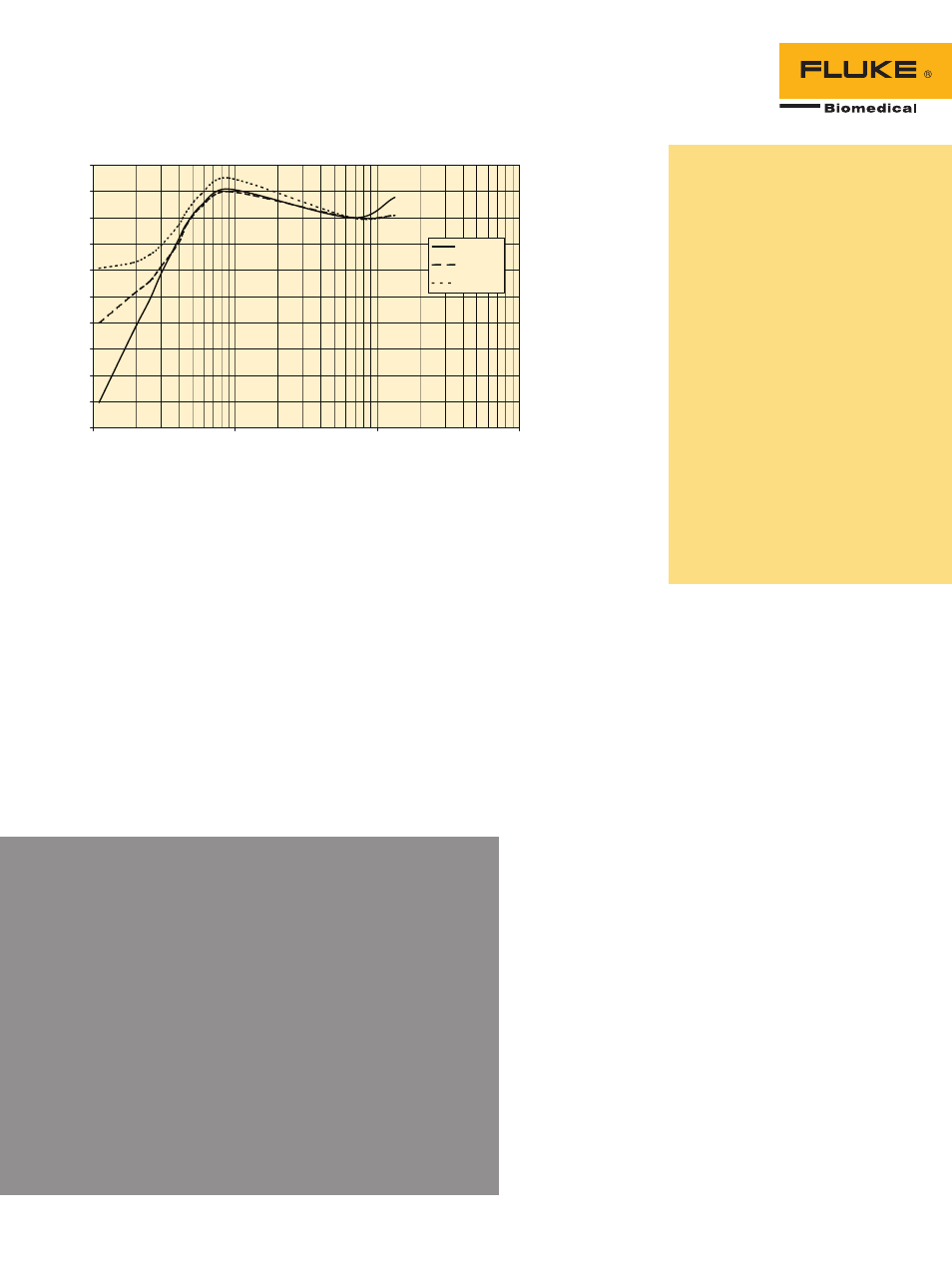
451B typical energy dependence
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
1.1
1.2
0
0
0
0
1
0
0
0
1
0
0
1
0
1
Effective (keV)
Indi
cate
d/
Act
ua
l
Slide - Open
Side
Slide - Closed
3 Fluke Biomedical 451B Ion Chamber Survey Meter with Beta Slide
Fluke Biomedical.
Better products. More choices. One company.
Fluke Biomedical
6045 Cochran Road
Cleveland, OH 44139-3303 U.S.A.
Fluke Biomedical Europe
Science Park Eindhoven 5110
5692EC Son, The Netherlands
For more information, contact us:
In the U.S.A. (800) 850-4608 or
Fax (440) 349-2307
In Europe/M-East/Africa +31 40 267 5435 or
Fax +31 40 267 5436
From other countries +1 (440) 248-9300 or
Fax +1 (440) 349-2307
Email: [email protected]
Web access: www.flukebiomedical.com
©2007-2013 Fluke Biomedical. Specifications subject
to change without notice. Printed in U.S.A.
7/2013 3095534D_EN
Modification of this document is not permitted
without written permission from Fluke Corporation.
About Fluke Biomedical
Fluke Biomedical is the world’s leading manufacturer of quality
biomedical test and simulation products. In addition, Fluke Biomedical
provides the latest medical imaging and oncology quality-assurance
solutions for regulatory compliance. Highly credentialed and equipped
with a NVLAP Lab Code 200566-0 accredited laboratory,
Fluke Biomedical also offers the best in quality and customer
service for all your equipment calibration needs.
Today, biomedical personnel must meet the increasing
regulatory pressures, higher quality standards, and rapid
technological growth, while performing their work faster and more
efficiently than ever. Fluke Biomedical provides a diverse range
of software and hardware tools to meet today’s challenges.
Fluke Biomedical Regulatory Commitment
As a medical test device manufacturer, we recognize and follow certain
quality standards and certifications when developing our products. We
are ISO 9001 and ISO 13485 medical device certified and our products are:
• CE Certified, where required
• NIST Traceable and Calibrated
• UL, CSA, ETL Certified, where required
• NRC Compliant, where required
