3B Scientific Air Cushion Plate User Manual
Page 24
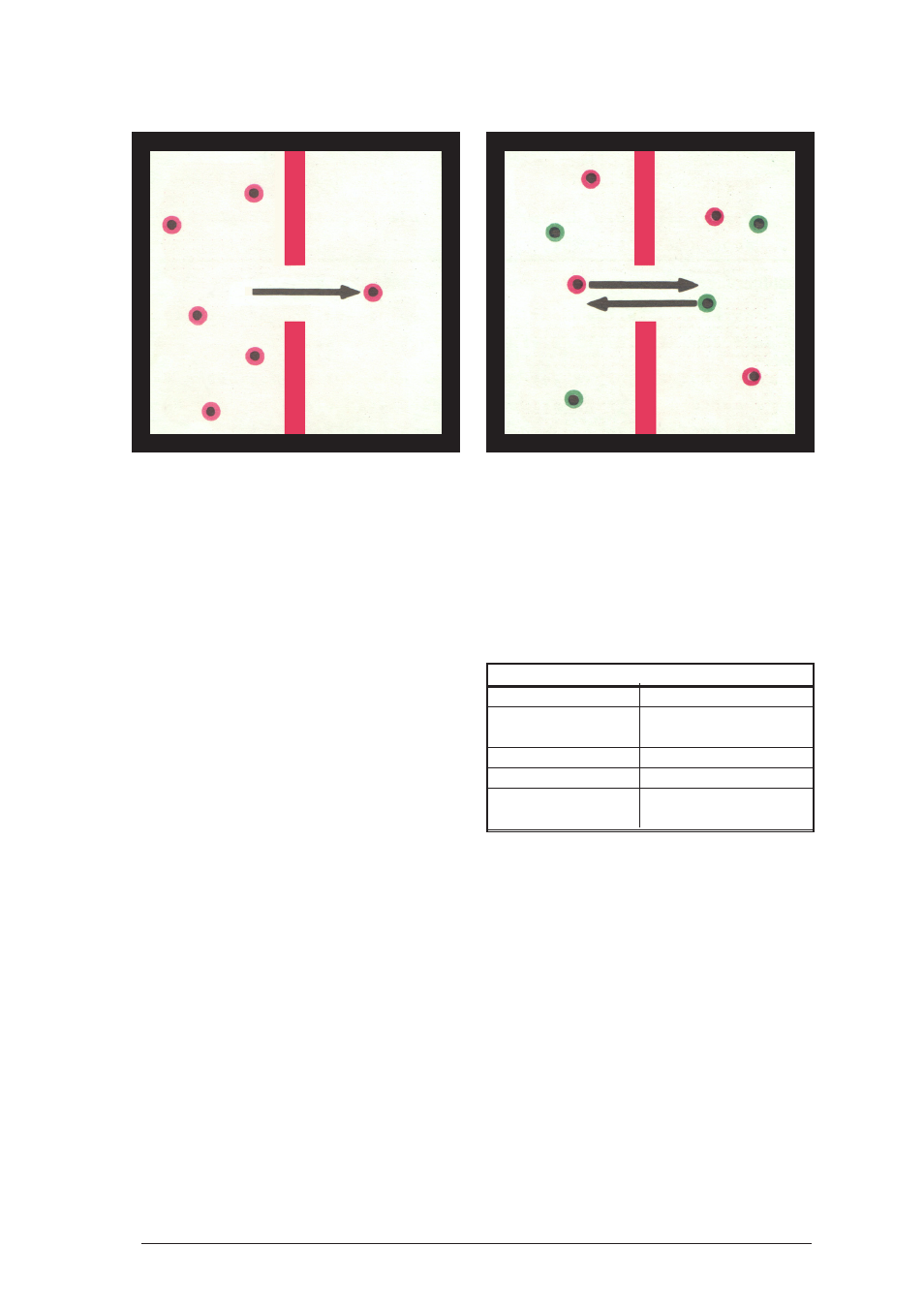
25
Physical Experiments on the Air-Cushion Table
in the other.
After turning the fan on, observe the movement
of the hover discs through the opening. Increase
the mean velocity of the hover discs by repeatedly
opening the impulse valve for a short time.
Result:
In all experiments hover discs move through the
opening at irregular intervals. As a result in the
first two experiments, each half contains
approximately the same amount of discs after
some time. In the last experiment the hover discs
penetrate the opening in both directions, so that
both types of discs mix. These procedures will
occur more rapidly the higher the mean velocity
of the hover discs is.
Interpretation:
The molecules of a gas can penetrate a porous
partition. If the gas is initially contained in one
chamber of a vessel with a porous partition, the
diffusion through the partition causes an equal-
ization of pressure so that eventually both cham-
bers contain the same amount of molecules. If the
two chambers of a vessel divided by a porous par-
tition contain different gases, these gases will mix
through the partition. Diffusion will occur more
rapidly the higher the temperature of the gases is.
2.1.16 Brownian Motion in a Gas
Components:
Air-cushion table with fan
Overhead projector
Magnetic barrier, long
2 Pieces
Magnetic barrier, short
2 Pieces
Hover disc, red
16 Pieces
Hover disc, blue
l Piece
Model simulation
Real Object
Model
Vessel containing
Experiment surface
the gas
of the air-cushion
Walls of the vessel
Magnetic barriers
Gas molecules
Red hover discs
Particle showing the Blue hover disc
Brownian motion
How to proceed:
Align the air-cushion table horizontally and attach
the magnetic barriers around the experiment
surface.
Arrange the red discs near the magnetic barriers
of the air-cushion table. Place the blue disc at the
center of the experiment surface.
Set the fan to a medium setting. Observe the
motions of the blue disc.
