Ivoclar Vivadent Helioseal Clear v.2 User Manual
Ivoclar Vivadent Equipment
Description
Helioseal
®
Clear is a light-curing, transparent fissure sealant.
Composition
Helioseal Clear consists of Bis-GMA and tri e thylene glycol dimethacrylate
(> 99 wt%). Additional contents are stabilizers and catalysts (<1 wt%).
Indication
Helioseal Clear is suitable for the sealing of pits, fissures and foramina caeca.
Contraindication
–
If the patient is known to be allergic to any of the material's ingredients.
–
If a dry working field cannot be established.
Side effects
In individual cases, contact allergies may occur. According to today's standard
of knowledge, systemic side effects are not known.
Interactions
None known to date
Preparatory steps
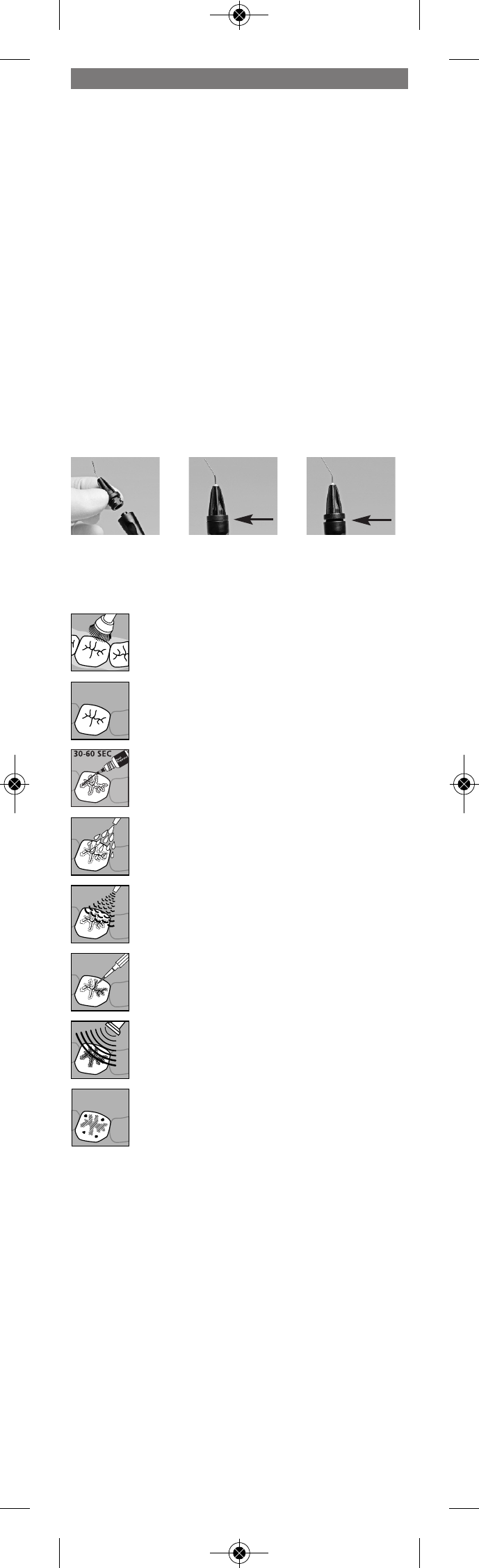
1. Remove the syringe cap by twisting it until it snaps and then pulling it off
the syringe.
2. Attach the applicator tip (Fig. 1) and twist it until it is flush with the
syringe (Fig. 2).
Step-by-step application procedure
1. Thoroughly clean the enamel surface to be sealed.
2. Isolate the working field, preferably with a rubber dam.
3. Apply an etching gel, e.g. Email Preparator, and let it
react for 30 to 60 seconds.
4. Rinse thoroughly.
5. Dry with water- and oil-free air. The etched enamel should
have a mat white appearance. Avoid contamination of the
etched surface with saliva.
6. Apply Helioseal Clear directly with the disposable cannula
or a disposable brush, and disperse.
7. Wait for approx. 15 sec. Then cure the sealant with a
suitable polymerization light (e.g. bluephase
®
) for
20 sec.
8. Check seal and occlusion.
Warning
Avoid contact of unpolymerized material with skin/mucous membrane or eyes.
Unpolymerized Helioseal Clear may cause slight irritation and, in rare cases,
may lead to a sensitization against methacrylates. Commercial medical gloves
do not provide protection against the sensitizing effect of methacrylates.
Special note
Helioseal Clear syringes must be used exclusively with the application tips pro-
vided. Other types of application tips may suddenly pop off during dispensing.
Storage
–
Close Helioseal Clear syringe immediately after use
–
Store material at 2–28 °C (36–82 °F)
–
Shelf life: see date of expiration
–
Do not use the material after the date of expiration
–
Syringes should not be disinfected with oxidizing disinfection agents.
Keep out of the reach of children.
For use in dentistry only.
This material has been developed solely for use in dentistry. Processing should be carried out strictly accor-
ding to the Instructions for Use. Liability cannot be accepted for damages resulting from failure to observe
the Instructions or the stipulated area of application. The user is responsible for testing the material for its
suitability and use for any purpose not explicitly stated in the Instructions. Descriptions and data constitute
no warranty of attributes and are not binding.
English
✗
✓
Fig. 2: right
Fig. 3: wrong
Fig. 1
Helioseal Clear_GI_61x245_Halb_637728_Rev0 21.03.
