Concentration vs ph, 5 ion selective features – Electro-Chemical Devices (ECD) HYDRA Ammonium User Manual
Page 25
HYDRA NH
4
+
-N
Page 17
3.5 ION SELECTIVE FEATURES
The Hydra C22 analyzer has several features unique to Ion Selective Electrode measurements,
Isopotential, Interfering Ion Correction and pH Correction. The measurement range of the sensors is
from some ppb level to some ppm level, XXX ppb – XXX ppm, not the traditional 0 – XXX common to
most measurements, there is no zero value. This is most evident when configuring the 4-20 mA outputs.
3.5.1 The Isopotential point
Isopot in the menu is the mV value of the specific sensor where changes in the temperature of the
solution do not change the output of the sensor. This is the base point for temperature compensation
correction. Most pH electrodes have an isopotential near pH 7, but each type of Ion Selective electrode
has a unique isopotential value. Each NH4+ and K+ electrode is supplied with an Isopot that must be
entered in the respective Set-Up Menu.
3.5.2 Potassium Ion Interference Correction
Potassium ions and Ammonium ions have a similar ionic size and the same electrical charge. If
Potassium ions are present in the measured solution then they will cause a positive Interference on the
Ammonium Ion Measurement. The corrections for the Interfering Ions are in the Ks and K+ comp screen
in the Set-Up menu. The Ks, potassium ion selectivity, is the correction factor, ppm NH
4
+
/ppm K
+
;
Example: (50 ppm K
+
) x (0.10 ppm NH
4
+
/ ppm K
+
) = 5 ppm NH
4
+
interference
With the Ks set at 0.10 (10 K
+
/ NH
4
+
) then 50 ppm potassium ion would cause the ammonium
measurement to read 5 ppm high. When the K+ comp line is On the C22 Analyzer uses the potassium
ion concentration from the Potassium Ion Electrode and the Ks factor to calculate the amount of
interference on the Ammonium Ion Electrode and subtracts it from the measured ammonium value
before displaying the NH
4
-N value.
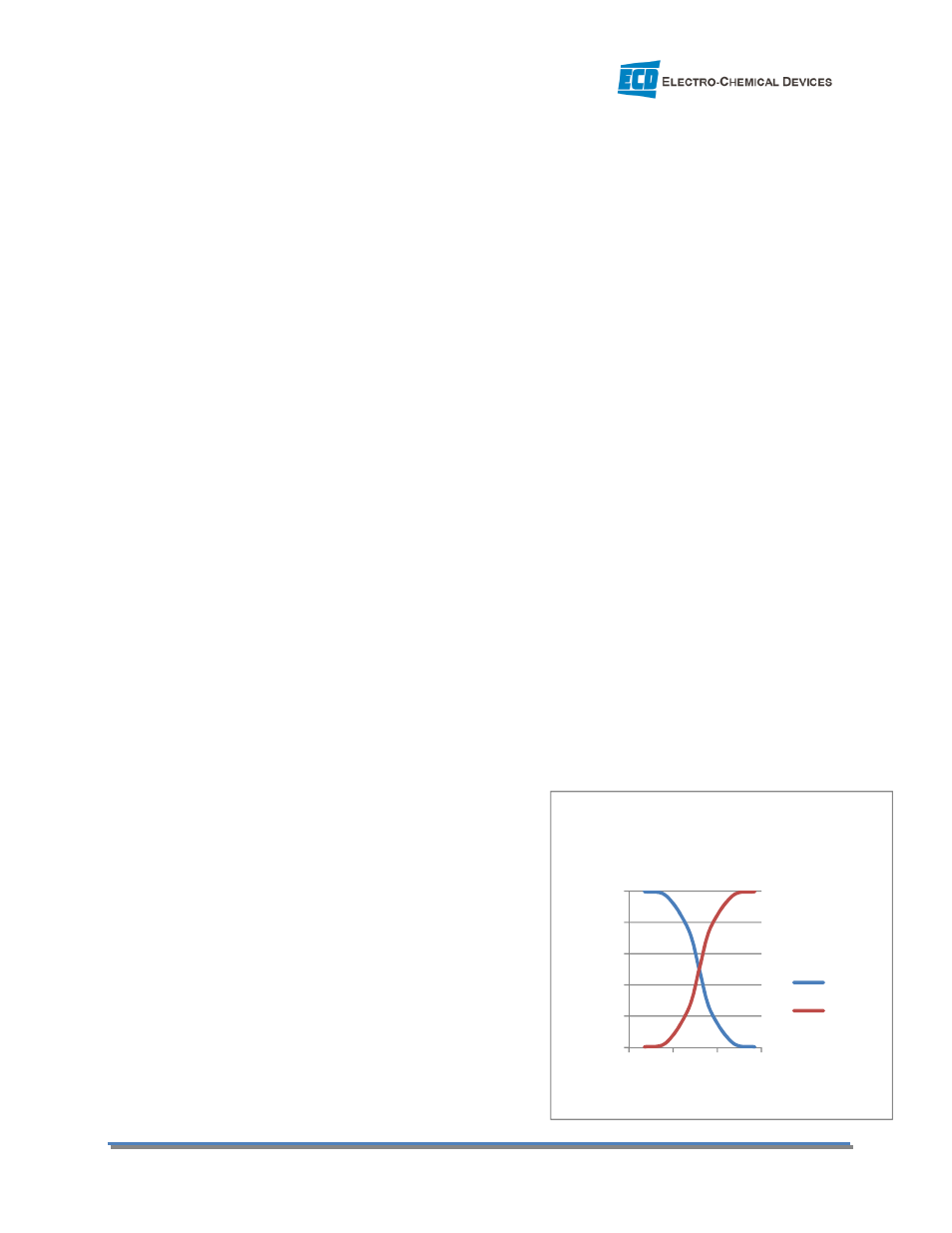
3.5.3 pH Correction
The Ammonium Ion Electrode only measures the ammonium ion (NH
4
+
) not ammonia (NH
3
). Ammonium
ion and ammonia coexist in a pH dependent ratio in
solution. At pH 9.24 the ratio is 1:1, at pH 7 nearly all of the
ammonia is in the ammonium state and at pH 11 it is nearly
all ammonia which is invisible to the sensor. The more acidic
values favor the NH
4
+
and the more basic values favor
ammonia gas, NH
3
. The pH Electrode measures the pH and
the HYDRA C22 Analyzer calculates the total NH
4
+
-N
concentration based on the pH vs. concentration profile.
The Dissociation should be On at all times.
0
20
40
60
80
100
6
8
10
12
%
pH
NH
3
/NH
4
+
% concentration vs pH
% NH4+
% NH3
